soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
synthesis of n-acetyl cysteine amide
I'm interested in synthesizing n-acetyl cysteine amide. 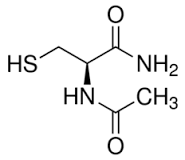
Haven't been able to locate much information online on how to do it.
React n-acetyl cysteine with thionyl chloride and then with ammonia?
Suggestions?
Thanks.
[Edited on 10-8-2015 by soma]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
it would be interesting if you tried a beckmann reaction to get the n-acetyl group.You might even get 100% of the correct stereoisomer
|
|
|
Nicodem
|
Thread Moved
10-8-2015 at 09:33 |
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
You will need to protect the thiol group to do the chemistry efficiently. Perhaps one way to get to your target is start with the disulfide dimer of
cysteine which is cystine. This can be bis-acetylated then converted to the bis-methyl ester via a Fischer esterification. Treatment of the bis-ester
with methanolic ammonia will give the bis-amide. Finally the disulfide can be reduced to the thiol providing your desired product.
A similar procedure would be di-acetylate cysteine (N and S), make the methyl ester and treat with methanolic ammonia. This will provide the amide and
cleave the S-acetate at the same time giving you your desired product.
By the way, using thionyl chloride to activate the carboxyl as you mention will lead to a mess, not the least of which is significant racemization of
the cysteine.
AvB
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Thanks.
Is there a name for the reaction converting the ester to the amide with methanolic ammonia? Haven't been able to find information on it so far.
[Edited on 11-8-2015 by soma]
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
We usually just call it ammonolysis. Basically, you dissolve your ester in methanol, cool it to about -20C and saturate it with ammonia gas. Securely
cap the reaction vessel and let it sit for a day or so at room temperature. Strip off the methanol and ammonia (carefully) and purify your product as
needed.
AvB
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
When you say "securely cap" - it builds up a pretty good amount of pressure? Suitable for glassware?
How about directly reacting the di-acetyl cystine with ammonia using heat?
For the final reduction -- using something like sodium borohydride or sodium?
Thanks again.
[Edited on 12-8-2015 by soma]
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Just found an interesting article on direct amidization of carboxylic acids using urea and microwave. http://www.cs.gordon.edu/~ijl/_lead_papers/Direct%20amidatio...
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Would the direct amidization using urea and microwave need a protected nitrogen?
Also found this: Synthesis of amides from unprotected amino acids by a simultaneous protection–activation strategy using dichlorodialkyl silanes. http://www.sciencedirect.com/science/article/pii/S0040403902...
Anyone have access to Tetrahedron Letters? This is Volume 43, Issue 50, 9 December 2002, Pages 9203–9207.
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
soma,
I've uploaded the file here:
http://www.filedropper.com/2002leeuwentetlettsynthamidesilan...
And yes, you would use sodium borohydride for the reduction.
Cheers!
[Edited on 19-8-2015 by bereal511]
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
By the way, what are you planning on doing with NACA?
If you make any progress, let us know. I'm interested in the synthesis as well.
- Bryan
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for the ref. I'm interested in its use in treating cataracts.
Researchers at Missouri University of Science and Technology are working with an antioxidant that could prevent or cure cataracts, macular
degeneration and other degenerative eye disorders. -- http://news.mst.edu/2012/09/antioxidant_may_prevent_even_c/
Also --
In the present human health scenario, implication of oxidative stress in numerous pathologies including neurodegenerative, cardiovascular, liver,
renal, pulmonary disorders, and cancer has gained attention. N-Acetylcysteine (NAC), a popular thiol antioxidant, has been clinically used to treat
various pathophysiological disorders. However, NAC therapy is routine only in paracetamol intoxication and as a mucolytic agent. Over six decades,
numerous studies involving NAC therapy have yielded inconsistent results, and this could be due to low bioavailability. In order to overcome the
limitations of NAC, an amide derivative N-Acetylcysteine amide (NACA) has been synthesized to improve the lipophilicity, membrane permeability, and
antioxidant property. Recent studies have demonstrated the blood-brain barrier permeability and therapeutic potentials of NACA in neurological
disorders including Parkinson's disease, Alzheimer's disease, Multiple sclerosis, Tardive dyskinesia, and HIV-associated neurological disorders. In
addition, NACA displays protective effect against pulmonary inflammation and antibiotic-induced apoptosis. Forthcoming research on the possible
therapeutic properties of NACA and its generics in the management of pathologies associated with extracellular matrix degradation and oxidative
stress-related inflammation is highly exciting. Superior bioavailability of NACA is likely to fulfill the promises of NAC as well as a molecule to
improve the endurance and resident time of bioscaffolds and biomaterials. Till date, more than 800 reviews on NAC have been published. However, no
comprehensive review is available on the therapeutic applications of NACA. Therefore, the current review would be the first to emphasize the
therapeutic potentials of NACA and its derivatives. -- http://www.ncbi.nlm.nih.gov/pubmed/23472882
[Edited on 21-8-2015 by soma]
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
Have you considered looking into N-acetylcysteine ethyl ester (NACET)?
I'm trying to look into the pharmacology of the compound, but I haven't turned up any results. The only reference I could find was this recent one on
its enhanced lipophilicity compared to NAC:
http://www.ncbi.nlm.nih.gov/pubmed/23000913
http://www.filedropper.com/2012giustarinibiochempharmanacetk...
The claims are a little dramatic, so I wouldn't take too much of that paper seriously. The real nice bit is that they mention 60% bioavailability of
NACET over the 5% bioavailability of NAC.
The advantage would be that it's relatively simple to synthesize in comparison to NACA, although it's worthwhile to note that there's been a lot more
work done on NACA on its metabolism and physiology compared to NACET (like I said, I've only found one reference). That being said, ethyl esters of
amino acids are known supplements and seem to be relatively safe.
Here's a quick and easy synthesis that I pulled up:
http://www.filedropper.com/1982bruiceproteinchemsynthnacethy...
One-step esterification with anhydrous HCl and ethanol as solvent.
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
I'm wondering if acetylating cystine would already protect the nitrogen, so using the silane wouldn't be necessary. You could do the ammonolysis using
urea, imidazole, microwave and then reduce.
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AvBaeyer  | You will need to protect the thiol group to do the chemistry efficiently. Perhaps one way to get to your target is start with the disulfide dimer of
cysteine which is cystine. This can be bis-acetylated then converted to the bis-methyl ester via a Fischer esterification. Treatment of the bis-ester
with methanolic ammonia will give the bis-amide. Finally the disulfide can be reduced to the thiol providing your desired product.
A similar procedure would be di-acetylate cysteine (N and S), make the methyl ester and treat with methanolic ammonia. This will provide the amide and
cleave the S-acetate at the same time giving you your desired product.
By the way, using thionyl chloride to activate the carboxyl as you mention will lead to a mess, not the least of which is significant racemization of
the cysteine.
AvB
|
Found this at http://www.google.com/patents/US20080200548
So much for patents.
EXAMPLE 1 Synthesis of N-acetyl Cysteine Ethyl Ester (Compound A)
N-acetyl cysteine (4.6 mmol) was added in portions to a cooled (e.g., 2-8° C.) solution of 2 ml thionyl chloride and 10 ml absolute ethanol. The
resulting mixture was refluxed at 40° C. for 1 hour and then the volatiles were removed in vacuo. The residue was dissolved in 10 ml of water and was
extracted twice with 20 ml of methylene chloride. The extract was dried under vacuo. The title compound was crystallized from petroleum ether
(fraction 40-60°) in 55% yield.
The resulting product has the following characteristics:
(a) Melting point of 90° C.
(b) Anal. calculated for C7H11NO3S:
Calculated: C, 43.9 H, 6.8
Found: C, 42.5 H, 6.0
(c) Thin layer chromatography in n-butanol/acetic acid/water (4/1/4): was carried out and the Rf value was Rf=0.91. The Rf value of the reactant,
N-acetyl cysteine is 0.78.
(d) Nuclear Magnetic Resonance (NMR) in deutarated trichloromethane (CDC13):
6.51, 0.7H
4.85, 1H, m
4.23, 2H, q, J=7.0
3.44, 0.4H, d, J=4.4
3.22, 2H, t, J=4.4
2.06, 3H, S
1.30, 3H, t, J=7.0
EXAMPLE 4 Synthesis of N-acetyl Cysteine Amide (Compound J)
Ammonia gas was bubbled through absolute dry ethanol at −70° C. (dry ice with acetone), for 10 minutes. N-acetyl cysteine ethyl ester (compound A),
163 mg (1 mmol) was added to the cooled ethanol/ammonia solution and ammonia was continued to bubble through the solution for additional 10 minutes.
Then, the solution was corked and was left at room temperature. After 16 hours, the flask was opened and access of ammonia and the ethanol were
evaporated under reduced pressure. The product was lyophilized. The yield was 98%.
The resulting product has the following characteristics:
(a) Thin layer chromatography in n-butanol/acetic acid/water (4/1/4) was carried out and the Rf value was Rf=0.70. The Rf value of the reactant,
N-acetyl cysteine ethyl ester is 0.91.
Alternatively, a solution of 20% piperidine (4 ml) in 16 ml DMF was added to Fmoc Rink amide AM resin (2 gram; 1.1 mmole amide) and the reaction was
allowed to proceed for 30 minutes. Ac-S-trityl cysteine (1.3 gram, 3.3 mmole) was added with TBTU (1.06 gram) followed by diisopropyl ethyl amine
(1.12 ml). The reaction was carried out for 2 hours. The resin was washed with methylene chloride (×6), and then a mixture of 1 ml silan/0.5 ml
water/19 ml of TFA was added. After 1 hour the resin was filtered washed with TFA and solvent evporated. The product was dissolved in water and
extracted with methylene choride. The aqueous solution was thereafter lyophyllized.
The resulting product has the following characteristics:
(a) Nuclear Magnetic Resonance (NMR)
4.45 t,1,j=6.96 Hz
2.81 ABX system, JAB=12.69, JAX+JBX=12.45Hz
2.00 s 3Hz
**********************************
I am planning to get around to this as soon as some other projects are finished.
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bereal511  | Have you considered looking into N-acetylcysteine ethyl ester (NACET)?
I'm trying to look into the pharmacology of the compound, but I haven't turned up any results. The only reference I could find was this recent one on
its enhanced lipophilicity compared to NAC:
http://www.ncbi.nlm.nih.gov/pubmed/23000913
http://www.filedropper.com/2012giustarinibiochempharmanacetk...
The claims are a little dramatic, so I wouldn't take too much of that paper seriously. The real nice bit is that they mention 60% bioavailability of
NACET over the 5% bioavailability of NAC.
The advantage would be that it's relatively simple to synthesize in comparison to NACA, although it's worthwhile to note that there's been a lot more
work done on NACA on its metabolism and physiology compared to NACET (like I said, I've only found one reference). That being said, ethyl esters of
amino acids are known supplements and seem to be relatively safe.
Here's a quick and easy synthesis that I pulled up:
http://www.filedropper.com/1982bruiceproteinchemsynthnacethy...
One-step esterification with anhydrous HCl and ethanol as solvent. |
Tried looking up the files at filedropper but they weren't there.
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
Are you still looking for a synthesis?
I found a rather simple one here by the Babcock Institute:
http://www.sumobrain.com/patents/wipo/Method-preparation-n-a...
[0019] /V-Acetyl Cysteine Methyl Ester:
[0020] A suspension of /V-acetyl-L-cysteine (32.6 g) in dry methanol (120 mL) under nitrogen was stirred for 15 minutes and treated dropwise with
concentrated sulfuric acid (0.8 mL) at room temperature with vigorous stirring. After 22 hours of stirring, the mixture was treated with water (25 mL)
and the volatiles were removed under reduced pressure. The resulting residue was diluted with ethyl acetate (200 mL), washed with aqueous saturated
sodium bicarbonate (150 mL) and the layers were allowed to separate.
[0021] The organic layer was separated from the aqueous layer and dried over anhydrous sodium sulfate. The aqueous layer was re-extracted with ethyl
acetate (2 x 100 mL). The combined organic extract was filtered and concentrated in vacuo to yield /V-Acetyl- L-cysteine methyl ester (24.1 g, 68%) as
a white crystalline solid: 1 H NMR (400 MHz DMSO- cfe) δ (ppm): 8.29 (d, 1 H), 4.39 (m, 1 H), 3.60 (s, 3H), 2.77 (dd, 1 H), 2.70 (dd, 1 H), 2.51 (s,
1 H), 1.84 (s, 3H); LRMS: 178.13 (M+H) + .
[0022] Scale-up Preparation of /V-Acetyl Cysteine Methyl Ester: To a suspension of N- acetyl-L-cysteine (162.7 g) in dry methanol (600 mL) under
nitrogen was added
concentrated H2SO4 (4 mL) drop-wise at room temperature with vigorous stirring. After 24 hours of stirring, the mixture was slowly treated with
saturated aqueous sodium bicarbonate solution (100 mL) and stirred for 1 hour.
[0023] The solvent was removed under reduced pressure, and the resulting aqueous portion was extracted with dicholoromethane (4 x 100 mL), dried over
anhydrous sodium sulfate, concentrated and vacuum-dried to afford the desired methyl ester product as an off- white solid (12
[0024] /V-Acetyl-L-Cysteine Amide (NACA):
[0025] /V-Acetyl-L-cysteine methyl ester (10 g) under a flush of nitrogen was treated with ammonium hydroxide (28% aqueous, 66 mL) over 10 minutes at
room temperature and stirred for 6 hours. The resulting solution was concentrated in vacuo and ethanol (100 mL) was added. The resulting solution was
concentrated again under reduced pressure at 48 °C, then subjected to high vacuum overnight to afford /V-acetyl-L-cysteine amide (NACA, 9.12 g) as a
white crystalline solid (m.p. 138 - 141 °C; Lit. 148 - 150 °C); 1 H NMR (400 MHz DMSO- cfe) δ (ppm): 7.89 (d, 1 H), 7.30 (s, 1 H), 7.01 (s, 1 H),
4.16 (m, 1 H), 2.64 (dd, 1 H), 2.52 (dd, 1 H), 1.74 (s, 3H); LRMS 163.13 (M+H) + .
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
I did the micirowave synth. The reactants formed a liquid as they were supposed to., after 100 seconds at 300W. II tried heating for a little longer
and a vapor started forming.
The reactants were bubbling for awhile but now there's a fairly clear lacquer.
To extract, one method dissolved the product in water and then used DCM. The reactants were n-acetylcysteine, urea, and imidazole.
I don't want to use DCM (and don't have any). I've got ethyl acetate, methanol, ethyl ether, hexane, naptha, and thf.
Any suggestions on how to extract?
Thanks.
[Edited on 3-2-2016 by soma]
[Edited on 3-2-2016 by soma]
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Actually, it's more like an oil than a lacquer.
I think the vapor may have been the urea? There are crystals on the side of the glass bowl that may have been from the vapor.
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
I didn't do so hot extracting with hexane or ethyl ether. I would recommend either ethyl acetate or methanol, although a patent somewhere suggests
methyl tert-butyl ether. Sigma Aldrich says it's pretty soluble in DMSO, so it's probably quite polar overall.
One problem that I'd been running into was that cysteine and cysteine-derivatives like to dimerize to cystine in oxygen. I've been getting around
that with nitrogen, so I suspect you might have quite a bit of your share of n,n-diacetyl cystindiamide. Also, depending on where you're getting your
n-acetylcysteine, it seems that there's a rather significant amount of n,n-diacetyl cystine in the mix.
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Do you have an HPLC setup? Wondering how you're testing it. Are you doing the microwave synth?
I've read that the S-S bond is fairly easy to cleave so maybe something like TCEP could be used?
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Further update:
The tlc showed that there was NAC amide produced. The standard seemed to dimerize after a bit and I saw the same dimer in the product.
|
|
|