Orsyn
Harmless

Posts: 26
Registered: 21-2-2015
Member Is Offline
Mood: :booM
|
|
NH4NO3 heat sensitivity
Hey all, my first post here.
I'm reducing some AN for some Aqua Regia, and I wonder how sensitive it is to heat?
Is it dangerous to reduce on an electric burner?
I read the MSDS and looking at the flashpoint, it seems reasonably safe, but I wanted to double check.
Thanks! BTW awesome forum, I look fwd to learning a poop-ton here..
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
It melts, then decomposes. Depending on what you do, and HOW MUCH, bad things can happen. Numerous hippies used to make nitrous oxide for giggles
doing this, most of them lived.
Describe precisely what you want to do, please provide references/where you found the information you are working from?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
greenlight
National Hazard
   
Posts: 755
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I have used an electric hotplate on low to medium heat to dry Ammonium nitrate for use in ANNM charges. It once accidentally started melting slightly
on the bottom but no fire or decomposition.
[Edited on 21-2-2015 by greenlight]
|
|
|
Orsyn
Harmless

Posts: 26
Registered: 21-2-2015
Member Is Offline
Mood: :booM
|
|
Quote: Originally posted by Bert  | It melts, then decomposes. Depending on what you do, and HOW MUCH, bad things can happen. Numerous hippies used to make nitrous oxide for giggles
doing this, most of them lived.
Describe precisely what you want to do, please provide references/where you found the information you are working from?
|
I should have been a bit more clear in that what I'm wanting to do is simply filter as many impurities out of an aqueous sltn. made from two mal-wart
cold packs and some tap water. I'm pretty sure these cold-packs (CA) are calcium ammonium nitrate.
The goal here is to have something I can nitrate HCL with so I can strip some Au and Pt off some scrap electronics.
So far I''ve just been dissolving the packs in water, I haven't started reducing yet. Yesterday I read the MSDS and decided I should double-check the
safety of this procedure, that was primarily inspired by this (I watch a lot of Nurd-Rage's channel as well as Nile Red's):
https://www.youtube.com/watch?v=Jt-676bfXRI
Thanks for the replies, I'll definitely keep the burner down and keep an eye on it for any signs of melting..
cheers
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
If you are just doing the same procedure in the video, there is a minimal risk of anything bad happening. By taking it off of the hot plate before
all of the water has boiled off, it essentially prevents the temperature from rising too high and the ammonium nitrate shouldn't melt. To be safe,
keep a thermometer in the mixture and if it starts rising very quickly from the temperature it was boiling at, remove it from heat.
|
|
|
greenlight
National Hazard
   
Posts: 755
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I dont think you will have any problems boiling it down as shown in your linked video. As long as you dont boil to dryness and then keep heating after
all the water is gone which would probably cause it to melt. Just keep an eye on it and maybe even just cool it right down when about 2/3 of the
water has boiled off to precipitate the AN.
Exactly what gdflp said. 
[Edited on 21-2-2015 by greenlight]
|
|
|
Orsyn
Harmless

Posts: 26
Registered: 21-2-2015
Member Is Offline
Mood: :booM
|
|
Would ammonia smell (gas) be a product of decomposition?
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Strange stuff to heat. It will melt/dissolve in it's own dampness.
If you can find a book by cornelius kelter (i think) called Fertizisers (I think).
Attachment: Storage_of_hot_ammonium_nitrate_solutions.pdf (713kB)
This file has been downloaded 408 times
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
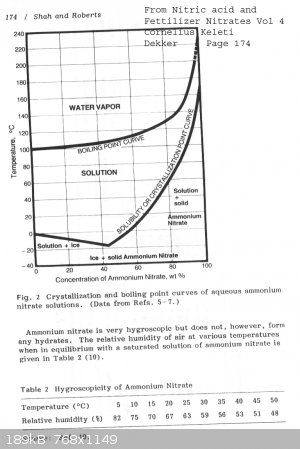
page from book
|
|
|