Ingram
Harmless

Posts: 4
Registered: 21-5-2006
Member Is Offline
Mood: No Mood
|
|
Home synthesis of HCl
Over the past few weeks I have been contemplating my first real chemistry experiment at my home. What I wanted to do was make a small amount of
hydrochloric acid. My original ideas were as followed:
Materials:
*Two glass holding containers (with the ability to be sealed)
*One AC/DC adapter
*Two carbon electrodes
*Distilled water
*Sodium chloride
*Rubber/plastic pipe
*A tupperware sort of deal
Setup:
IMAGE:
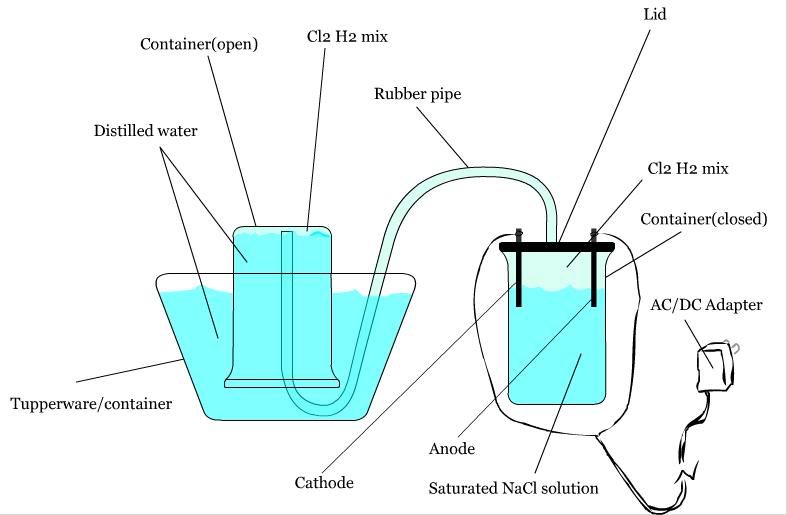
What I was planning on doing was having a concentrated sodium chloride solution in one of the two containers sealed, with the electrodes (connected to
the AC/DC adapter, of course) inside the solution. Then, attatch the pipe to the top of the container, pipe it under distilled water into the other
upturned container. When the AC/DC adapter is plugged in, what should happen is:
Na+ + 2Cl- --> Na + Cl2
2Na + 2H2O --> 2NaOH + H2
So, from the bold, you can see the diatomic hydrogen and chlorine gas that are produced. This will run up the pipe, and to the second container, and
will be over water, which should hopefully keep it somewhat pure, at which point I will have the two of them react to form HCl gas, cover the second
container, and then shake it to dissolve the HCl into the water.
So this is where my questions begin:
1) Is this a possible setup for making hydrochloric acid?
2) How explosive would the reaction between the hydrogen and chlorine be?
3) How could I get the chlorine and hydrogen to react? Do they need a spark, or can sunlight, as I have heard can, cause the two to react?
Thanks in advance for any information that you can give.
Dont panic.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
H2 and Cl2 is very voilent once initiated. There is a demo which consists of filling a thick glass tube with a stoichiometric mix of the gasses in
the dark, stoppered, and then a 'photo' is taken of the tube when it is in the light and the flash initiates the explosvie reaction blowing out the
stoppers.
Here is Tacho's experiment with something very similar. https://sciencemadness.org/talk/viewthread.php?tid=2154#pid2...
Yes, your method is feasible, but you need some work on how you are going to react the H2 and Cl2.
EDIT: You should use separate tubes for the hydrogen and oxygen coming off the cell, otherwise an explosion could blow your electrolysis cell as well
as your reaction vessel.
[Edited on 22-5-2006 by rogue chemist]
|
|
|
Ingram
Harmless

Posts: 4
Registered: 21-5-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by rogue chemist
H2 and Cl2 is very voilent once initiated. There is a demo which consists of filling a thick glass tube with a stoichiometric mix of the gasses in
the dark, stoppered, and then a 'photo' is taken of the tube when it is in the light and the flash initiates the explosvie reaction blowing out the
stoppers.
Here is Tacho's experiment with something very similar. https://sciencemadness.org/talk/viewthread.php?tid=2154#pid2...
Yes, your method is feasible, but you need some work on how you are going to react the H2 and Cl2.
EDIT: You should use separate tubes for the hydrogen and oxygen coming off the cell, otherwise an explosion could blow your electrolysis cell as well
as your reaction vessel.
[Edited on 22-5-2006 by rogue chemist] |
Yes, that probably wouldnt be a bad idea to use different tubes. Would it be possible to simply react the two together as they came out with the
light, creating an exothermic, but non-explosive reaction?
[Edited on 22-5-2006 by Ingram]
Dont panic.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Read the entire thread I linked you to, regular light is not good enough, but UV works.
|
|
|
Mechaton
Harmless

Posts: 22
Registered: 24-3-2006
Member Is Offline
Mood: No Mood
|
|
Wait a second, I have a tube from my chlorate cell running to a pipe going through the wall of my house. When I start the cell a decent amount of H2
and Cl2 bubble out of the cell and through that pipe. Now, I haven't seen any gouts of flame blasting out the side of my house...
|
|
|
Ingram
Harmless

Posts: 4
Registered: 21-5-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by rogue chemist
Read the entire thread I linked you to, regular light is not good enough, but UV works. |
EDIT: I did read that ultraviolet light can trigger it, but upon further reading, I see that this could or could not work. Thank you for pointing out
my error.
[Edited on 22-5-2006 by Ingram]
Dont panic.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
No or only negligible amounts of Cl2 forms if the anode is not separated from the catode where the hydroxyde anions form (Cl2 is soluble in
hydroxydes, forming hypochlorite or chlorates if the temperature is higher).
So, in short, this setup would not produce a mixture of chlorine and hydrogen unless there is a porous membrane separating the electrodes.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
kyro8008
Harmless

Posts: 32
Registered: 24-8-2005
Location: UK
Member Is Offline
Mood: No Mood
|
|
This method looks to me like a bit of a pain in practice.
Why not heat solid NaHSO4 with solid NaCl which forms HCl and run this HCl through a tube straight into distilled water. This is not a perfect method
and can be a pain but much easier than the other method I would think.
NaHSO4 can easily be bought from hardware stores/hydrophonics stores, etc as "pH Down".
I used to make my HCl this way until I found 20 Litres of 36% for £12!
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Another serious issue is that Cl2 dissolves quite well in water. In the beaker, which you have upside down, if a mix of H2 and Cl2 arrives over thre,
the Cl2 will dissolve in the water, while the H2 does not. So, the mix of gases in the beaker will be very low in chlorine. Taking into account all
the other issues (like hypochlorite/chlorate formation) I do not think that this method is feasible.
If you want to make it feasible, make Cl2 and H2 in separate containers. A syringe for the H2 would be nice. Connect a thin glass tube to the syringe,
slowly press the syringe, such that the H2 comes out. Light it and then move the tube into the Cl2 gas. The H2 will continue burning in the Cl2 gas.
This is nice as a curiousily and would make a decent experiment, but for preparative purposes it also is too cumbersome.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Does hypochlorite/chlorine solution attack H2 at all?
Maybe with some Pt or Pd catalyst? (Further complicating things eh?)
Tim
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Once I made quite satisfied AlCl3 quite dry from Al foils and HCl gas....
my gaseous HCl was produced from sodium chloride and sulphuric acid:
2 NaCl (s) + H2SO4 (aq) ---> 2 HCl (g) + Na2SO4 (aq)
The HCl so produced can be dried in some ways...however I've not much drying agents...so ask someone about this.
Hope this helps
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
try kings chemistry survival guide - has exactly what you are trying to do - last experiment in the book.. 
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
| Quote: | Originally posted by jimmyboy
try kings chemistry survival guide - has exactly what you are trying to do - last experiment in the book..  |
Thats about the last thing to recommend to anybody you don´t hate.
This book is almost COMPLETE BULLSHIT! Be warned! This does not work this way. Even Uncle Fester wasn´t telling so much nonsense never. Well almost
never.
"Kings Chemistry" is dangerous bullshit just a notch above the "Anarchist Cookbook".
|
|
|
Ingram
Harmless

Posts: 4
Registered: 21-5-2006
Member Is Offline
Mood: No Mood
|
|
Well what would you suggest for a porous membrane? Other than the NaHSO4 + NaCl, it seems like it would be the most plausible method for me to do
this. Thanks in advance.
And also, thanks for the warning about King's Chemistry Survival Guide. I do know that the anarchist cookbook is horribly inaccurate though. Good
comparison. 
[Edited on 25-5-2006 by Ingram]
Dont panic.
|
|
|