LifeisElemental
Harmless

Posts: 39
Registered: 18-7-2014
Location: United States
Member Is Offline
|
|
A Safer Aqua Regia?
Hi all,
I realize I very recently posted a thread similar to this but I feel it is different enough to justify its own discussion.
I have recently built a fume hood and have heard the advice that one of the best ways to use a fume hood is to not use it at all. For example,
neutralizing toxic gasses instead of just letting them be sucked through.
Recently, I have been considering using aqua regia to reclaim gold from old computer parts and I would like to know if there is a more ideal
alternative than just placing the parts in a beaker and letting the NO2 loose.
I have included a picture of one particular thought I had... perhaps a large funnel could be placed over the reaction beaker with a hose leading to
another funnel containing an alkali solution?
Any suggestions or comments on this?
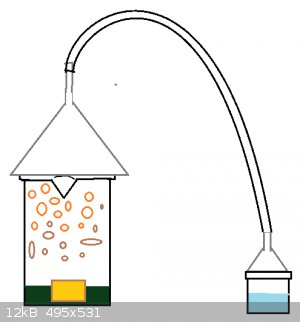
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Worth trying. And recover the N as nitrate, eventually.
[Edited on 6-10-2014 by blogfast25]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by LifeisElemental  | Hi all,
I realize I very recently posted a thread similar to this but I feel it is different enough to justify its own discussion.
I have recently built a fume hood and have heard the advice that one of the best ways to use a fume hood is to not use it at all. For example,
neutralizing toxic gasses instead of just letting them be sucked through.
Recently, I have been considering using aqua regia to reclaim gold from old computer parts and I would like to know if there is a more ideal
alternative than just placing the parts in a beaker and letting the NO2 loose.
I have included a picture of one particular thought I had... perhaps a large funnel could be placed over the reaction beaker with a hose leading to
another funnel containing an alkali solution?
Any suggestions or comments on this?
|
Observation 1: You will still need to use a fume hood, or similar excellent ventilation, since the acid bath will producing chlorine, nitric oxide,
and nitrogen dioxide, and no scrubbing system you devise is likely to scrub them all. Such a system is perhaps possible, but that would require
development and testing.
Observation 2: The current arrangement won't remove any NO to speak of since nitric oxide is highly insoluble in water. To react any NO with a
detoxing chemical it has to bubble up through your scrubbing solution. NO will be partly oxidized to NO2 which can be trapped in water more easily,
but not all of it.
Observation 3: There is no advantage in using a funnel. A flat lid with a hole for the tube would be easier to work with. Better gas control would
result from using two holes, one for an air stream going in (small aquarium pump perhaps) and one going out to flush the gas into the scrubbing
solution.
Observation 4: Bleach solution might be better. NO reacts with hypochlorite, and commercial bleach contains NaOH as well.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
You could just add a little hypochlorite to a hydroxide solution. The NO would react with the hypochlorite and the chlorine would regenerate the
hypochlorite by reacting with the hydroxide, while the NO2 reacts with hydroxide to make nitric acid and nitrous acid.
|
|
|
LifeisElemental
Harmless

Posts: 39
Registered: 18-7-2014
Location: United States
Member Is Offline
|
|
Thanks for the suggestions!
So here is a new image of a possible way about this.
Pictured is a thick black rubber stopper which is stopping the beaker containing aqua regia. I realize this may be a horrible idea and would like some
input. If this would not work then I would simply use a RBF although getting the computer parts inside would be a challenge.
If there are no objections, the tubing I would be using is a braided vinyl.
I will further consider the 2nd hole with the pump as suggested by careysub.
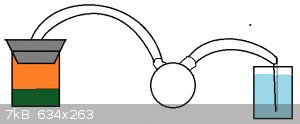
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Unless you're planning to process huge quantities of material which would make these drawings superfluous the environmental impact is very small and
your own safety more important. Do it in a hood and make yourself a good fume hood or shop for one that you can get cheap and install. I have a small
plexiglass one that I would let you have cheap.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LifeisElemental  |
So here is a new image of a possible way about this.
Pictured is a thick black rubber stopper which is stopping the beaker containing aqua regia. I realize this may be a horrible idea and would like some
input. If this would not work then I would simply use a RBF although getting the computer parts inside would be a challenge.
If there are no objections, the tubing I would be using is a braided vinyl.
I will further consider the 2nd hole with the pump as suggested by careysub.
|
i am impressed at your ingeniuty,but i think that you should look at this method first
see this video .
in this nurdrage makes nitric acid in three ways
in the first way he generates NO2 by any nitrate salt,HCl and Copper.he puts the salt and copper in a beaker,places the beaker(with the
salt +copper) in a water bath,adds the HCl and quickly inverts a bigger beaker over the first beaker(with the salt +copper).what happens is that the
NO2 generated is forced to dissolve in the water and he gets impure and dilute nitric acid.when the reaction gets too vigorous(when a lot
of NO2 is generated) the bigger beaker starts shaking and wobbling so he places another flask with cold water on the top of the bigger
beaker to keep it steady and help the NO2 to condense faster.
you could do a similar thing by putting your beaker with aqua regia in a water bath(you could put some bleach or NaOH in the water to absorb the
gases as the other members said.
as all the gases generated(Cl2,NO2,etc) are heavier than air ,they will eventually settle down and dissolve in the bleach or
alkaline water.
alternatively you could replace nitric acid with hydrogen peroxide(but it has to be strong,at least 30%,not the 3% that you can buy in medical shops)
as nurdrage says in this video
all the best
[Edited on 7-10-2014 by CuReUS]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Also be aware that nitric acid and nitrous oxides eat away the rubber of stoppers! That's why when distilling nitric acid, an all-glass apparatus is
required.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
you might be able to do it electrically like this http://www.youtube.com/watch?v=arlYPz3EP7A by replacing sulphuric acid with HCl ,if gold sulphate is not soluble
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
or you could try lugol's solution
http://www.sciencemadness.org/talk/viewthread.php?tid=21769
|
|
|