(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
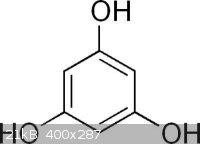
Phloroglucinol synthesis
hi all etc.
I was looking for a way to make Phloroglucinol. (1,3,5-tri hydroxy benzene)
my first action was to search online ! 
but to my wonder, almost no result is found there.
wiki says : start from benzene, and go from nitro - amine - base.
but I don't wanna deal with explosives.
another suggestion i found on other forums goes from 1,3,5 tri chloro benzene - amine - base.
which is only a suggestion proposed there that works (maybe only) in theory.
OK, my first & sub-question is : Can this tri-chloro-benzene be converted to tri-hydroxy without using high pressure ?
(high pressures + high temperature is what they use in industrial scale. but i can't prepare that pressures required  , but maybe it can be done with catalysts+high temperature in absence of high pressure.
any ideas ? ) , but maybe it can be done with catalysts+high temperature in absence of high pressure.
any ideas ? )
guess what, hereby I discount my second and main question so you can focus on one only. 
any paper, patent, textbook, pre-1900 book, and of course ideas are welcomed.
TnX
Dibrainamine
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
You might want to check out the following: PHV ANALYITIC US2005/165256 A1, 2005 and J. Am. Chem. Soc., 2005, 127 (15), pp 5332–5333
|
|
|
Nicodem
|
Thread Moved
5-9-2013 at 05:12 |
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kavu  | | You might want to check out the following: PHV ANALYITIC US2005/165256 A1, 2005 and J. Am. Chem. Soc., 2005, 127 (15), pp 5332–5333
|
thanks, but 2 little problems :
I could not find the first reference you sent.
&
the second one is for biosynthesis, my emphasize is on lab synthesis.
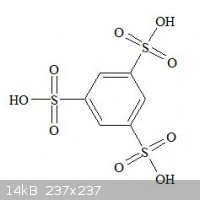
anyway, another idea of mine was to react 1,3,5 tri sulfonic acid benzene with NaOH and heat.
but first I don't know ( ) how to synthesize the trisulfonic acid. ) how to synthesize the trisulfonic acid.
all I know is : it can't be made from toluene sulfonation as it yields only para-sulfonic acid.
So my first question (asked earlier) can be interpreted as this : how to make 1,3,5-trisulfonic acid benzene ?
TnX
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
You can't under any reasonable conditions. To introduce even two meta-sulfonic acid groups onto benzene requires oleum instead of concentrated
sulfuric acid. One route to phloroglucinol passes through trinitrobenzene which is itself very difficult to make. However, you can convert TNT into it
with two steps. TNT however typically requires oleum to produce as well.
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
So it seems it's better to drift my questions line a little bit more .
maybe I can find other 1,3,5-tri X benzene structures,and convert them to Phloroglucinol.
Another Question arises here in my miserable mind  : :
what functional groups can be converted to hydroxy group, (of course on aromatic rings) ?
up to now i know the following : NO2, NH2, SO2OH
TnX
Dibrainamine

RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Interesting Procedure
hehe, it seems I'm almost answering my questions, shame on you people ! 
OK, it was just a joke. [I'm not that demanding creature who I always seem to be !]
I found this :
OrgSyn.Org - CV3P0288
Q1. What can be used instead of BaCO3 ?
Q2. what is the best choice for reducing that COOH to phenol ?
I know it's a stupid way to ask about carboxylic acid reductions, but the reason is, though I am a very shrewd chemist and know everything  (joking again) , I've heard the chemistry of aromatic rings is a bit different that
other situations. so I wanted to ask if ordinary COOH reducing systems would work well here in aromatic ring or not. (joking again) , I've heard the chemistry of aromatic rings is a bit different that
other situations. so I wanted to ask if ordinary COOH reducing systems would work well here in aromatic ring or not.
Please Help Me On This One.
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Theres a route from bromobenzene via nitration andreduction. The resultant 1,3,5-triaminobenzene is rdily hydrolysed (think of it as the tri-imine
tautomer). Method is given in a journal, search google for "novel synthesis of phloroglucinol", its in a paper to do with magnesium binding to human
serum albumin or something? Provided at ScienceDirect. Overall yield is not too bad, I recall something like 60%th?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by (Brain)2NH  | another suggestion i found on other forums goes from 1,3,5 tri chloro benzene - amine - base.
which is only a suggestion proposed there that works (maybe only) in theory. |
Which theories say it would work? The theory of the elimination-addition mechanism (aka "benzyne mechanism") that you imply by the conditions you
later mention, say that the reaction would unlikely give the 1,3,5-triaminobenzene as a predominant product. The benzyne substitution would require
heating in anhydrous ammonia to a couple of hundreds °C under huge pressure. Isomeric products and other side products (assuming reaction completion
could even be achieved) would be statistically much more likely than 1,3,5-triaminobenzene.
Besides, it is bad manners to not cite sources. Give the proper reference for these "other forums" so that we can judge the reliability of the data by
ourself.
Phloroglucinol can be prepared from 1,3,5-tribromobenzene by the CuI catalyzed substitution with sodium methoxide in methanol/DMF to first give the
1,3,5-trimethoxybenzene which is then demethylated with aq. HCl at room temperature. 1,3,5-Trichlorobenzene is said to stop at the
3,5-dimethoxychlorobenzene at the same conditions. See: Synthetic Communications 1974, 4, 35-43.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
you're right nicodem, I thought it's not even worthy to mention the forum there, because on that forum no one replied to him, and I thought maybe
everyone thought it to be a foolish idea,
And the reason yet I mentioned the idea itself was to have s.th to prove I have UtFSE. 
OK, i'll post the forum asap i find it.
Thanks, N.
But how the Sodium Methoxide is made ? (Other than Na + MeOH)
as this is gonna work, would it matter if I use other RONa stuff, instead of MeONa ?
I mean can this (replacement of MeONa) cause lower yields in hydrolysis process ?
TnX
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Did you totally miss my post?
http://www.ncbi.nlm.nih.gov/m/pubmed/14565560/
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Thanks DJF90 for your responces
but I don't have access to it.
can only see abstracts if i'm lucky enough 
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Why don't you ask in references section...
Just this once:
Attachment: 1-s2.0-S0731708503002206-main.pdf (226kB)
This file has been downloaded 951 times
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
thanks for the attachment DJF, same trinitro method though.
anyway TnX
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Same trinitro method as what? Everything discussed in this thread is trihalo or trisulfonate. Nicodem mentioned trinitrobenzene. What I've done is
shown you the viable experimental route that I was talking about.
[Edited on 8-9-2013 by DJF90]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
As Wiki said, my friend
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Maybe that there are other possible methods...
1°) Starting from the aromatic ring...
From 1,3,5-trimethylbenzene...
-acidic permanganic oxydation of the methyl groups into carboxylic acids... yielding 1,3,5-benzo-trioic acid
Ar-CH3 -H(+)/KMnO4 --> Ar-CO2H
-transamidation with urea into 1,3,5-benzo-trioic acid triamide
2 Ar-CO2H + NH2-CO-NH2 --> 2 Ar-CO-NH2 + H2O + CO2
-Hoffman Rearrangement of the triamide into 1,3,5-triamino-benzene
Ar-CO-NH2 --> Ar-NH2 + CO2
-hydrolyse and expulsion of NH3 by concentrated NaOH and heat...
2°)Starting from alkylic chain and aromaticising
Via aromatic cyclisation of 3-nitro-pyruvic acid methyl ester in neutral or mild acidic media...yielding
1,3,5-methyltricarboxylate-2,4,6-trinitrobenzene...
-Hydrolysis of the three methyl ester and thermal decarboxylation into 1,3,5-Trinitrobenzene
-Reduction of the trinitrocompound into triamino compound
-Hydrolysis and expulsion of NH3 by concentrated NaOH and heat...
Maybe 3-hydroxy-pyruvic acid esters will condense directly into a phenolic compound (like 1,3-dihydroxypropanone does into polyphenols - base of
artificial suntanning) then you would get 1,3,5-trihydroxy-2,4,6-tricarboxyalkyl-benzene.
-Hydrolysis of the three alkyl ester and thermal decarboxylation into 1,3,5-Trihydroxybenzene.
3-hydroxypyruvic acid esters should be reacheable from 3-chloropyruvic acid...itself abtainable from pyruvic acid or ester and acidic halogenation...
Theorically 2-hydroxy-ethanal should condense spontaneously into phloroglucidol... just like 2-chloroethanal would into trichlorobenzene and
2-nitroethanal would into TNB...but there is a chance the process goes also partly into polymerization... and not only into cyclotrimerization
n Y-CH2-CH=O --> (-CHY-CHOH-)n --> (-CY=CH-)n + n H2O
[Edited on 17-9-2013 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Reflux with NaOH, and a little CuI and ammonia added as a catalyst, for 1-2 hours.
http://www.organic-chemistry.org/abstracts/literature/090.sh...
[Edited on 17-9-2013 by AndersHoveland]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Thanks all, really useful ideas.
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|