ppk77
Harmless

Posts: 3
Registered: 11-5-2012
Member Is Offline
Mood: No Mood
|
|
Dehalogenation of fluorobenzene
Hi,
I'm looking for information/a method on reversing the halogenated of a benzene ring, for example removing the fluoride from Fluorobenzene to revert it
back to simple benzene.
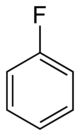
to

Many thanks in advance for any information.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Why not do a Google on 'benzene dehalogenation' or 'benzene defluorination'?
|
|
|
ppk77
Harmless

Posts: 3
Registered: 11-5-2012
Member Is Offline
Mood: No Mood
|
|
I found one which described the catalytic dehalogenation of benzene using palladium and whilst being far too complex for my level of practical
understanding in the lab - I don't actually have any palladium or really wish to purchase any for a small experiment.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I suspect that you are looking at reactions like that.
Fluorine is quite hard to remove.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Fluorine is typically nearly impossible to remove from organic molecules. But there are at least two ways of doing it. strong
fluorine abductors (such as BF3 or SbF5) can work through the transient formation of the carbenium ion ion, but this can be very hazardous
and impractical. (remember that the resulting alkylating agents from the reaction can be deadly, it would be much like working with magic methyl)
CH3F + SbF5 --> CH3[+] + SbF6[-]
CH3[+] + acetate[-] --> methyl acetate ester
I also remember reading about another reaction that was very recently invented in the journal of chemical engineering, but unfortunately I cannot find
the exact reference.
http://www.rsc.org/chemistryworld/News/2011/June/23061103.as...
| Quote: |
researchers [Alan Goldman] at Rutgers University in New Jersey have cracked the problem by using a catalyst based on an iridium centre clamped between
two bulky dialkylphosphino groups - a so-called pincer-ligated complex.
|
In conclusion, there is no simple and easy way to dehalogenate fluorobenzene. It would be far easier to just buy or make some benzene, rather than try
to make it from fluorobenzene.
To destroy fluoro-organic compounds, they typically must be destructively oxidized, then hydrolysed with water to recover the fluoride ions.
[Edited on 12-5-2012 by AndersHoveland]
|
|
|
Nicodem
|
Thread Moved
12-5-2012 at 03:05 |
ppk77
Harmless

Posts: 3
Registered: 11-5-2012
Member Is Offline
Mood: No Mood
|
|
Thank you very much - looks like it's back to the drawing board, thank you again for the info.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
NaK alloy (which is a liquid at room temperature) would probably be able to reduce fluorobenzene, but I am not sure if benzene would be the resulting
product.
Sodium hydride, in the presence of catalytic quantities of nickel acetate and sodium isopropylate, can reduce aryl fluorides (such as p-fluorotoluene)
under mild conditions. 90% conversion in 3 hours, 100% within 7 hours. It is important that the nano-sized particulate NaH be used, for maximum
surface area.
"An effective method for the hydrodehalogenation of organic halides", Haiqing Li, Shijian Liao, Yun Xu, Daorong Yu and Zhiming Qiao
[Edited on 16-5-2012 by AndersHoveland]
|
|
|