| Pages:
1
2 |
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Ozone generator.
Some time ago, I have built an ozone generator capable of filling the ambient with that smell. I would guess I had a 0,5% ozone coming out of the
“corona chamber”. The best part was to watch the corona inside (I had a window). Some beautiful visual happened there.
The main problem was overheating of the transistors. The apparatus could only work for a few minutes. I used the classic 2 transistor (2N3055)
circuit. I used huge dissipators.
Recently there was some discussion on the V2O5 thread about ozone generators. I began to have that “Close-encounter
–of-the-third-kind-fixed-idea”. You remember the 77’s movie: Richard Dreyfus gets fixed on sculptures of the mountain (potato puree
and so on). This happens to me with my projects. BTW does this happen to anyone else or should I look for medical help?
So I took a look at my old fellow countryman Sam Barros' page and gess what I found:
http://www.powerlabs.org/flybackdriver.htm
A one transistor driver! I thought: that’s soooo easy to make...
So I made it in 10 minutes and, to my surprise, works very well, with no overheating. This thing can run for hours! I used a rectified 12V
transformer, so I estimate the voltage applied in about 17V.
So now I’m planning to do some research on the best cheap corona discharge chamber.
Out of SHEER ENVY I might try to mix ozone and SO2 from a sulfur candle and post the results before axehandle!
I attach a picture of the flyback circuit.

|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
That's very nice of you. I already built an ozone generator though, out of a drinking glass, some Al foil and a 9kV NST. But I won't pursue
the SO2 + O3 --> SO3 + O2 route unless all else fails. That includes trying a Pt catalyst as well. I'll get a very
<b>expensive</b> piece of Pt wire in 2 weeks at the earliest, so you
have plenty of time beating me to it. piece of Pt wire in 2 weeks at the earliest, so you
have plenty of time beating me to it.
And yes, you are insane. I get fixed ideas too, and I'm a certified whacko. Logically, that means you are one too. 
I'd recommend an NST instead of a flyback, though --- it's wattage is much higher 
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by axehandle
(snip)
And yes, you are insane. I get fixed ideas too, and I'm a certified whacko. Logically, that means you are one too. 
|
That damm mercury! I knew it!
| Quote: |
I'd recommend an NST instead of a flyback, though --- it's wattage is much higher  |
Well, maybe, but I'm 97% sure that wattage is not important to get ozone. You need high voltage, but very very little current.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Maybe we should call you Tacho the mad Hatter then. Blame the mercury all you like but if you are 97% sure by my math that gives you 3% common sense.
;D
In a well built system power is the limiting factor. You are making a high energy molecule from low energy ones, and wasting a fair amount of energy
on the way. A commercial generator I based my math on for the nitric acid numbers consumed 600W and produced 30g of ozone an hour. The concentration
of ozone was 6% in oxygen.
For the classic setup of a silent electric discharge a neon sign transformer would work very badly, prodicing something like 500 times less ozone than
a 10W flyback operating at 20KHz and the same voltage into a small area (read conventional sized) silent discharge.
Though a corona discharge will produce less ozone per watt, you can even use DC, so its only how much power the transformer can produce and how large
an area of discharge you can make that really matters. Keeping things cool, including the discharge, may require a very large box.
One way that might work to see if you are generating useful amounts of ozone would be to dissolve a tiny amount of an organic black dye in water, and
bubble the ozone in until it goes clear. I'm open to better suggestions though.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Thank you so much!
I learned a lot from your post and I feel much more enlightened about this subject now!
We are very lucky to have someone like you to teach us how to do things in the proper way!!
Keep up with the good job, old pal !!
Marvin, Marvin... I’m not biting your bait!
|
|
|
Organikum
resurrected
    
Posts: 2344
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
The attached .pdf might be of interest here. 
Attachment: ozone_generator.rar (37kB)
This file has been downloaded 1408 times
|
|
|
Organikum
resurrected
    
Posts: 2344
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
"Doppelfeldozonröhre"
aka: "doublefieldozonetube"
German patent DE886896 (attached)
The patent is in german but the drawing is quite self-explaining. They claim doubled yields of ozone by this design - the idea is convincing: There is
the outside as the inside of the highvoltage/highfrequency tube used - doubled efficiency - easy.
If there are problems in understanding the patent - ask and I will translate the main part.
Attachment: DE886896.djvu (51kB)
This file has been downloaded 1087 times
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
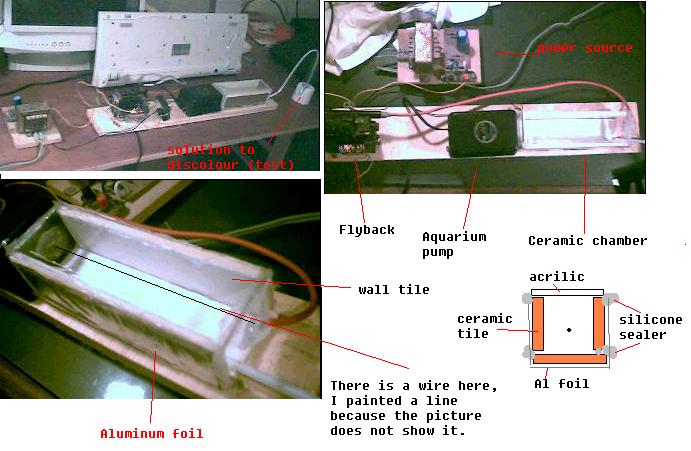
This is the new version of an old ozone generator I've built.
It turns water with a drop of blue ink from deep blue to almost clear in about two minutes.
It doesn't have the beautifull visual effects my old one had, but it seems to be more efficient in producing ozone.
It avoids the complicated glassware.
Thanks you for the input Organikum.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Marvinator.
*chuckles*
With some very dodgy math my wild guess would be not above a gram of ozone a day.
The only thing I can suggest, would be to dilute some 3% peroxide to a known lower concentration, and see how much of that is needed to clarify some
of the ink solution.
Since the ink is organic its quite possible it is being oxidised multiple times, thus making the error rather large but I still cant think of a better
way.
How well do you think the acrylic will stand up to the ozone?
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Oh! Marvin, Marvin, you noticed!
I’m soooo honored!
You think the acrylic won’t hold to the ozone? Well, you should see what happened to the latex tube I used in the output of my last generator! Do
you know any uses to latex goo? I'm sure you do!
I don’t care what happens to the acrylic.
I have so much to learn from you! Please, please, keep teaching me!
BTW, I did use the ink discolouring test. Thanks for that.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Without knowing too much about the ozonizator, i´m able to type in a few words in google and provide a link...maybe that helps a bit.
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
I also read that electrolysis of cold concentrated sulfuric acid is used in industry...
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
Well gee golly BASF.......
that's MUCH easier than the aluminum foil in a cup method, maybe I'll whip one up between breakfast and lunch! 
Edit "no offense intended eh! just acerbic humor"
[Edited on 5-4-2004 by Hermes_Trismegistus]
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
The potassium iodide->Iodine as a ozone measure is new to me! I like that.
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
and probably....
you could use any halide salt to halogen as a quantitative test
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Maybe, but iodine has strong color and low volatility(sp?). Chlorine does not make sense and bromine will evaporate quickly.
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
I guess it would be dependant on availibility.
Flexibility is always a good thing. Chlorine could be an odour/colour (of emitted gas) dependant test at it's most basic level, although I'm
sure there are other smple ways to monitor chlorine evolution.
In fact, the reaction itself might be useful for other things.
note* I'm not disputing the suitability of KI for quantitative analysis. It is, of course, the reigning champion for many procedures.
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
A general test for oxidizers is KI/starch paper, slighty moistened with dilute HCl. In the presence of an oxidizer Cl is liberated which liberates
Iodine which reacts with the starch to make a distinctive blue or black stain. Ozone should liberate the Chlorine from HCl and set things in motion. I
have seen it work with organic peroxides, nitrates, hydrogen peroxide, and bleach. I have not tried it with Ozone, but I could guess it would work.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Guess what? I did a test with KI solution and another with NaBr solution. KI goes from clear to tan in about 2 minutes. Nothing happens to NaBr.
It seems ozone is not a strong enough oxidizer to release bromine.
Shame. It would be a nice way to make elemental bromine.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
What is wrong with bubbling chlorine through to get the bromine?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hermes, yes its not simple, but on the other hand it is producing over 2 orders of magnetude more ozone than the corona wire chamber, and this is
still an order below what would be needed to make N2O5 from a 1kw arc furnace NO2 source.
Tacho, thanks are not needed, a small temple in my honor will be quite sufficiant.
You might not care about the plastic, but if it is reacting this will be reducing the output of ozone. Teflon tape might help.
If you can standardise some thiosulphate the reaction with iodine followed by titration will be a much better way of determining ozone. Iodine itself
is not particually strong coloured (for the concentrations used) but iodine/soluable starch peforms very well.
To try for bromine acidify the bromide solution first with HCl.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Now, that’s a beautiful post Marvin!
It expresses ideas, points of view and even has sense of humor.
Don’t you hate when people are rude, abusing and patronizing? Doesn't it spoil the fun when people express their point of view in an arrogant
‘I know all” way?
We are lucky not to have such people here!
I like the HCl idea very much. But lets start with a little shrine.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
No, HCl+NaBr does not work. I bubbled it for 20 min.
It was a good idea, though.
I presume that, if any bromine was formed due to ozone, it was carried away by the air stream.
I added some H2O2 (20%) to the solution and plenty of bromine was formed. Not enough to precipitate, but it turned the solution brown.
rogue chemist, there is nothing wrong about using chlorine. Have you ever done it?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Yes, once. I had a tiny(10mL) bottle of 10%KI solution from an old chem set. A few seconds later after bubbling Cl through I got a small amount of
I2. When I posted earlier I had misinterpreted the earlier posts and I some how thought the thread had turned into one on extracting halogens.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
I'm back!
But what is this junk?
| Quote: |
For the classic setup of a silent electric discharge a neon sign transformer would work very badly, prodicing something like 500 times less ozone than
a 10W flyback operating at 20KHz and the same voltage into a small area (read conventional sized) silent discharge.
Though a corona discharge will produce less ozone per watt, you can even use DC, so its only how much power the transformer can produce and how large
an area of discharge you can make that really matters. Keeping things cool, including the discharge, may require a very large box.
|
Conclusion: An NST would work best, but in the same time an NST wouldn't work best?
<b>Come on</b>, Marvin, even you <b>must</b> be able to screw with logic better than this. You don't even manage to
confuse <i>me</i>, which normally is very easy.
BTW: Very nice setup, Tacho. The acrylic "problem" is easily solved by replacing the plastic with sandblasted glass, glued in place by a
glue that passivises when oxidized. But I'm sure you've figured that out already.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Wellcome back axe!
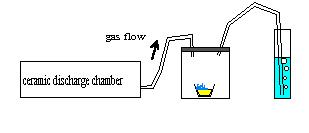
I just found out that my marvinator is not good enough to make H2SO4. You don't have to live in fear anymore!
I burned about 3 grams of sulfur in a 100ml glass flask, in the output line of ozone, bubbling the result gas in water. pH only went to 5 after about
15 minutes. Guess is just sulfurous acid. Any decent ammount of sulfuric acid would make my 10ml of water have a much lower pH.
I attach a drawing if anyone feels lucky:
|
|
|
| Pages:
1
2 |