| Pages:
1
2 |
Crazy_Chemist
Harmless

Posts: 19
Registered: 22-7-2019
Location: Europe
Member Is Offline
Mood: Radioactive
|
|
Best way to extract sodium hypochlorite?
Detergents with 2.4% sodium hypochlorite are sold here, and I need to extract it. I tried to boil it and got a powder, which developed chlorine when I
dripped hydrochloric acid on it.
But it says on Wikipedia that it decomposes at 101 ° C, so what did I get in the jar?
If I let it stand and evaporate, should it work? How stable is sodium hypochlorite really?
https://en.wikipedia.org/wiki/Sodium_hypochlorite
Amateur chemist, just for fun!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You made a mixture of sodium chloride and sodium chlorate. Upon heating hypochlorite decomposes into these two compounds.
|
|
|
Texium
|
Thread Moved
9-3-2022 at 23:04 |
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
You can buy 20 to 30 percent sodium hypochlorite as pool chlorinator. Or you can make it by mixing solutions of sodium sulfate and calcium
hypochlorite and filtering out the calcium sulfate leaving a sodium hypochlorite solution.
Concentrating sodium hypochlorite is not easy.
|
|
|
sauveurdumonde
Harmless

Posts: 33
Registered: 13-7-2021
Location: Canada
Member Is Offline
|
|
I recall reading that hypochlorite powders tend to have sensitive explosive tendencies. It may not be very easy to store anhydrous.
"There is a shadow of a nation behind you, the hope of a people... yet it may not matter. The Divide still stands against us."
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why?
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
swimming pool chlorine powders or ground up tablets with HCl are good for a chlorine gas generator
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
trichloroisocyanuric acid sold as chlorine shock or quick chlorine, tablets/granules react with NaOH to form sodium hypochlorite
calcium hypochlorite cannot be bought in europe- GENERALLY.
depending on the use you may also electrolyse NaCl to form it in situ
|
|
|
Crazy_Chemist
Harmless

Posts: 19
Registered: 22-7-2019
Location: Europe
Member Is Offline
Mood: Radioactive
|
|
Well, need and need, I just like to produce new chemical to have in my homelab  But...
But...
I dont think Im going to store it at ahome...
So, I think I'm going to the pool-store instead.
When I need chlorine gas, I usually use HCl and KMnO4.
Amateur chemist, just for fun!
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
NaClO is quite unstable. It never is sold as a solid. The solid does exist though, but it only is a lab curiousity, which fairly quickly decomposes.
Commercial solutions are limited to appr. 15%, usually less (12...13%). If the concentration is raised, then you see two competing decomposition
reactions. In one reaction there is disproportionation into NaCl and NaClO3, and in the other there simply is decomposition with production of O2. The
latter reaction can be enhanced severely by transition metal impurities. E.g. if you add a few drops of a cobalt salt solution to a concentrated
solution of NaClO, then the solution starts bubbling/foaming (production of O2).
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | NaClO is quite unstable. It never is sold as a solid. The solid does exist though, but it only is a lab curiousity, which fairly quickly decomposes.
Commercial solutions are limited to appr. 15%, usually less (12...13%). If the concentration is raised, then you see two competing decomposition
reactions. In one reaction there is disproportionation into NaCl and NaClO3, and in the other there simply is decomposition with production of O2. The
latter reaction can be enhanced severely by transition metal impurities. |
Disproportionation is promoted by heating. How do strong NaClO solutions behave on cooling - gentle cooling (deep freeze) and harsh cooling (like
liquid nitrogen)?
What would happen if frozen NaClO solution is allowed to stand at low temperature with but out of direct contact with a strong desiccant like CaO?
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I like problem solving challenges,
lateral, diagonal and upside-down thinking.
But potentially explosive substances in glassware is a poor choice.
(in my opinion)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Sodium hypochlorite pentahydrate is stable at refrigerator temperatures according to wiki.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
That's not a very efficient way of generating
chlorine. You'd be much better off finding either trichloroisocyanuric acid (TCCA), sodium dichloroisocyanurate (NaDCC) or calcium hypochlorite at a
hardware or pool store, and using that in combination with HCl to generate chlorine. At least one of those should be readily available for you and
cheap. Save your permanganate for better things.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I fully agree with Texium. KMnO4 is way to valuable for making Cl2.
Best is TCCA or Na-DCCA. I do not like Ca(ClO)2, because the resulting Cl2 is not very pure. It also produces ClO2 (you can see that the solution gets
a more intense color than one should expect from Cl2 alone). The ClO2 most likely is due to chlorate impurity of the Ca(ClO)2, which almost always is
present, especially if the material is somewhat older. A similar thing I also observed with somewhat older bleach as a source of Cl2.
The major disadvantage of using TCCA or Na-DCCA is that poorly soluble cyanuric acid is formed in the reaction, which easily leads to excessive
foaming. The Cl2-gas, however, is very pure (disregarding water vapor).
[Edited on 11-3-22 by woelen]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by chornedsnorkack  | How do strong NaClO solutions behave on cooling - gentle cooling (deep freeze) and harsh cooling (like liquid nitrogen)?
What would happen if frozen NaClO solution is allowed to stand at low temperature with but out of direct contact with a strong desiccant like CaO?
|
The hydrates are made by cooling etc. Frozen bleach would have a hard time evaporating.
I did the math and don't see it...more efficient based on HCl maybe, but HCl is really cheap. Permanganate generates more Cl per gram of oxidant and
the cost per gram of Cl is pretty much the same, not that TCCA has ever been sold locally outside of pool specialists. Permanganate (sold in about
half the hardware stores here since forever) has no storage issues, very much unlike the others.
The product(s) of the reaction depends on the amount and strength of acid (and sometimes temperature).
[Edited on 12-3-2022 by S.C. Wack]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Uh what?
You mean OTC or what?
verrückt und wissenschaftlich
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by S.C. Wack  | Quote: Originally posted by chornedsnorkack  | How do strong NaClO solutions behave on cooling - gentle cooling (deep freeze) and harsh cooling (like liquid nitrogen)?
What would happen if frozen NaClO solution is allowed to stand at low temperature with but out of direct contact with a strong desiccant like CaO?
|
The hydrates are made by cooling etc. Frozen bleach would have a hard time evaporating.
|
The vapour pressure of water is:
at +20 Celsius - 2339 Pa
at 0 Celsius - 611 Pa
ice at -20 Celsius - 103 Pa
So when a frozen hydrate is stored in a closed vessel but out of contact with a powerful desiccant, like CaO, P4O10 or concentrated H2SO4 (all of whic
would react with NaClO if in contact, but are even less volatile than water), then dehydration of hydrates would be one competitive reaction.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
That would be a serious disappointment to those who make freeze dryers.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
In other words, it would have a hard time evaporating.
If what we said had anything to do with operating freeze dryers.
[Edited on 12-3-2022 by S.C. Wack]
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by S.C. Wack  |
I did the math and don't see it...more efficient based on HCl maybe, but HCl is really cheap. Permanganate generates more Cl per gram of oxidant and
the cost per gram of Cl is pretty much the same, not that TCCA has ever been sold locally outside of pool specialists. Permanganate (sold in about
half the hardware stores here since forever) has no storage issues, very much unlike the others. |
I did more
math, and I still see it.
Reaction of potassium permanganate with HCl:
2 KMnO4 + 16 HCl —> 2 KCl + 2 MnCl2 + 5 Cl2
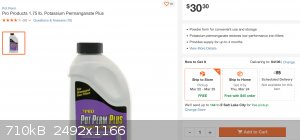
2.5 moles of Cl2 per mole of KMnO4. Permanganate is not regularly sold in any hardware store I've been to. The product "Pot Perm
Plus" is generally only obtainable by special order. If I wanted to get it from my local Home Depot, I would have to order it to the store to pick up.
Other stores don't offer it at all. It was the same case where I lived in Texas. Maybe other locales have it OTC. So that's 794 grams for $30.30, or
about $6 per mole, giving us a theoretical yield of $2.41 per mole of chlorine gas. With the caveat that permanganate isn't actually
OTC.
Now let's take a look at TCCA:
C3N3O3Cl3 + 3 HCl —> C3N3(OH)3 + 3 Cl2
3 moles of Cl2 per mole of TCCA. I could buy this at my local Lowe's in the store today if I wanted to:
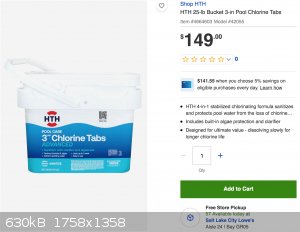
While it's much more expensive upfront, that's for 11.34 kg of 94% TCCA, or 10.66 kg of pure TCCA. This comes out to about $3.25 per mole, giving us a
theoretical yield of $1.08 per mole of chlorine gas.
While researching this, I was rather surprised to find that pool chemicals seem to be less available now than in years past when I bought them for my
lab. I couldn't find NaDCC on any sites, for instance, while I know that I had bought some from Lowe's or Home Depot in-person in 2015 in an
affordable 5 lb container of granules. Otherwise I would have included that in the price comparison too. It's always worth keeping in mind that
availability of OTC chemicals is constantly shifting, and some things that we take for granted may be phased out for unclear reasons.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by Texium  | | So that's 794 grams for $30.30, or about $6 per mole, giving us a theoretical yield of $2.41 per mole of chlorine gas.
|
My calculations were based on my (half perhaps, I'd have to actually go check) cost, not your needlessly expensive figure (the $20 at amazon is still
not the lowest that comes up with a search...their 5# @ $40.34 was used for the 93.6% TCCA)
On topic, I suspect that distillation with a high enough vacuum could concentrate dilute bleaches to a point where a hydrate could be crystallized out
by cooling.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by S.C. Wack  | Quote: Originally posted by Texium  | | So that's 794 grams for $30.30, or about $6 per mole, giving us a theoretical yield of $2.41 per mole of chlorine gas.
|
My calculations were based on my (half perhaps, I'd have to actually go check) cost, not your needlessly expensive figure (the $20 at amazon is still
not the lowest that comes up with a search...their 5# @ $40.34 was used for the 93.6% TCCA) |
My “needlessly
expensive figure” was based on what is available at hardware stores, which I used because you seemed to find it important to point out that
permanganate is easier to find OTC than TCCA (maybe it is in Cornworld, but here it’s certainly not). The price I quoted is for Home Depots across
the US. Maybe you have some special hardware store in Cornworld that sells it for half the price of the competition. Lucky you, if that’s the case.
The best price I can find online for permanganate is this: https://www.fleetfarm.com/detail/filter-mate-potassium-perma... which with the $8 shipping they offer does come out to about half the price of the
Home Depot stuff. Still, that only puts it roughly even with TCCA.
Overall, I still think TCCA is a better choice unless you have local access to much cheaper permanganate. TCCA has the advantage of not leaving you
with manganese waste to dispose of too.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by S.C. Wack  |
On topic, I suspect that distillation with a high enough vacuum could concentrate dilute bleaches to a point where a hydrate could be crystallized out
by cooling. |
My thoughts as well. You might even use a freeze drying apparatus for this.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by Texium  |
So that's 794 grams for $30.30, or about $6 per mole, giving us a theoretical yield of $2.41 per mole of chlorine gas. With the
caveat that permanganate isn't actually OTC. |
Quote: Originally posted by S.C. Wack  | | My calculations were based on my (half perhaps, I'd have to actually go check) cost, not your needlessly expensive figure |
I was off. The OTC product is actually $28.48. For 4.75#. But only until the sale is over, then it'll be $32.
You mean like what I said?
That product you found might be the same thing but with a different label, and (getting to the actual point) also be exactly what I have in stock from many years back, magically stable. Now you can put your skepticism to the test?
So how about finding a similar price for TCCA and recalculating, now that you've proven that permanganate is in fact cheaper. Personally if I was
going to special order something I'd go with the lowest price, esp. if it was to my door. And I'd buy a lot of it if it was useful and stable for
decades. What to do with Mn+2...yeah it's like radioactive waste and can't be isolated from solution, it's totally useless uh huhm, unlike cyanuric
acid which is so useful for...stabilizing Cl in pools.
I've used permanganate many times to make calculated amounts of Cl, even hypochlorite. Nothing wrong with it.
[Edited on 14-3-2022 by S.C. Wack]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It has.
Removal of water, as the vapour from a frozen material is (1) what the plan here was and (2) how a freeze dryer works.
Whether you remove it with a vacuum pump or a desiccant isn't a huge difference.
Obviously the pump works better but, if your idea that cold stuff won't evaporate were true, then freeze dryers wouldn't work.
They do.
It isn't.
|
|
|
| Pages:
1
2 |