smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Electrolytic Reduction of Unsaturated Beta-carbolines
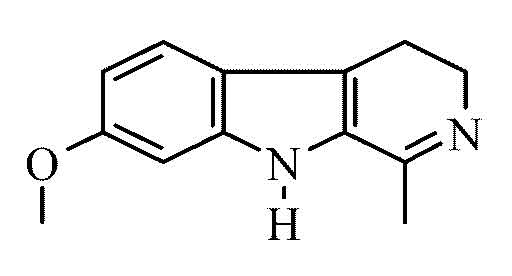
So essentially I'm looking at this unsaturated C=N bond and pondering. It has been reported in literature to be reduced via Zn and H+ with heat and
several other classic routes. Though I just got this electrolysis power supply a power cord and I think this sounds like too much fun to not
experiment with.
I have also read that pyridine has been reduced to piperidine using H2SO4 or TsOH, I think with a lead anode, and sufficient amperage per cubic cm.
So I'm really not seasoned with organic chemistry hence why this is in the beginners sub-forum. I am worried that the methoxy would undergo oxidation.
I probably shouldn't even be working on this due to a substantial lack of electrochemical and organic knowledge. Though it sounds like fun and I'm
sure there's a way to make this work. If anyone has any possible complications, hints or ideas it would be appreciated.
Here's what I'm thinking:
Use the sulfate salt of harmaline dissolve in minimal water. Add an excess of H2SO4. Use lead based solder(primarily lead) for electrodes, run 5 volts
through it. Try to monitor the reaction(if there is one...) by UV light looking for color changes(if even evident).
[Edited on 12-3-2011 by smaerd]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Sorry I can't help with the topic on hand but I would look more into the 3-ethoxy variations of this substance along with the 2-alkyl-pyridine analogs
if thats how you would lable it.
The Esters of the pyridine analogs have shown strong anti-depressent/ anxiolytic properties. When the major drug companys come to there senses and put
help over money this will replace SSRI's very quickly.
I may have a reduction in my notes that your looking for but im not sure if I have an electrochemical route. PM me for more information as this is a
bit off topic for this thread.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There are numerous reagents that can reduce imines to amines (NaBH4, silanes, Na2S2O4, metal dissolving reductions, hydrogenations over Pd-C or Raney Ni, CTH hydrogenations, etc.),
but if you want to try some electrochemical reduction then I suggest you to use some other substrate for tests. You can test the electroreduction on
something like nicotinate esters or amides (which can be made from niacin).
You will need a lead cathode, a graphite anode, a current supply, a semi-permeable membrane and a multimeter. Stirring during the reaction is also
advised and unless you try this on a couple of mmol scale, you will also need a cooling coil in the reaction mixture. The building of the apparatus
from common materials was already discussed at the forum.
You will also need to do a literature search, obviously.
PS: Where did you get harmaline? If you isolated it from Peganum harmala seeds, then you should know that the standard isolation procedure of the
poorly soluble hydrochlorides, as copypasted from an old article, actually gives a mixture of three alkaloids (the will be three spots on TLC, one
fluorescent). Also, why on earth would you want to reduce harmaline, such a nice and bioactive compound, to something as useless as tetrahydroharmine?
Check the price of harmaline at Si*ma (80 EUR/g!) and then reconsider about using that as a test substrate for such experiments! Use nicotinic acid
derivatives instead or whatever as cheap as that. You will also learn more as you will have to make an ester or amide first.
Quote: Originally posted by smaerd  | It has been reported in literature to be reduced via Zn and H+ with heat and several other classic routes.
...
I have also read that pyridine has been reduced to piperidine using H2SO4 or TsOH, I think with a lead anode, and sufficient amperage per cubic cm.
...
So I'm really not seasoned with organic chemistry hence why this is in the beginners sub-forum. |
I thought you opened the thread in the Beginnings section only because you were too lazy to cite the articles you talk about. It is not about how
seasoned you are, it is about the scientific method!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Well perhaps I got a little ahead of myself I haven't isolated pure harmaline yet. I did start from Peganum Harmala seeds, but plan on doing some
column chromatography after playing with possible eluents via TLC in the near future.
The goal of the experiment is merely novelty  . Cheap starting materials,
relatively non-toxic procedures, good practice, etc. I'm really interested in beta-carbolines themselves for reasons sedit has touched on above.
Besides reducing CuSO4 is fun for a while but this power supply wants to have more fun. . Cheap starting materials,
relatively non-toxic procedures, good practice, etc. I'm really interested in beta-carbolines themselves for reasons sedit has touched on above.
Besides reducing CuSO4 is fun for a while but this power supply wants to have more fun.
That's a very good call on testing the nicotinate esters first. Your post has helped me a lot to better shape the idea's further. My apologies for
not posting references I have them all downloaded in .pdf's and the one some-how doesn't include the author or the journal? So I made this kind of
scatter-brained. I'll dig them up and edit the original post.
|
|
|
|