| Pages:
1
2
3
4 |
boilingstone
Harmless

Posts: 8
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
Taken straight from wikipedia:
"Ozonolysis of alkynes generally gives an acid anhydride or diketone product, not complete fragmentation as for alkenes. A reducing agent is not
needed for these reactions. The exact mechanism is not completely known."
If in this reaction, R = halogen is in scope, then ozonolysis of the dihaloacetylene should yield the oxalyl halide.
I have a paper on the ozonolysis of acetylenes if anyone is interested
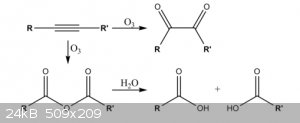
[Edited on 27-8-2018 by boilingstone]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by boilingstone  | Taken straight from wikipedia:
"Ozonolysis of alkynes generally gives an acid anhydride or diketone product, not complete fragmentation as for alkenes. A reducing agent is not
needed for these reactions. The exact mechanism is not completely known."
If in this reaction, R = halogen is in scope, then ozonolysis of the dihaloacetylene should yield the oxalyl halide.
I have a paper on the ozonolysis of acetylenes if anyone is interested
[Edited on 27-8-2018 by boilingstone] |
I am interested. I'm looking at a few proposed mechanisms for the reaction (it is typically performed at dry ice temperatures with non-halogenated
alkynes), and they don't show what happens to the singlet oxygen radicals... apparently, peroxide polymers can be produced as a byproduct. I bet the
workup is exciting.
Potassium permanganate can similarly oxidize alkynes to diketones, and the reaction can be performed at room temperature in DCM... at least for
alkynes where R and R' are carbon chains.
Dichloroacetylene can probably be prepared from tetrachloroethene, either by reducing it directly to dichloroethyne or reducing it to trichloroethene
and dehydrohalogenating it with a strong base.
[Edited on 27-8-2018 by JJay]
|
|
|
boilingstone
Harmless

Posts: 8
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
From what I've read about dichloroacetylene, I would have to strongly advise against its synthesis. According to wikipedia (again) dichloroacetylene
is extremely toxic, pyrophoric, and may detonate at its boiling point. It seems there is a good reason it was mentioned in "The War Gasses...".
While dibromoacetylene suffers from similar issues, apparently these issues are less severe.
again, reference "The War Gasses..." I posted last time for more info
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
First, one may wish to investigate a claim of a successful photochlorination path to (COCl)2, which has been previously presented on SM, see http://www.sciencemadness.org/talk/viewthread.php?tid=8291 ..
------------------------------------------
Found a photolysis path reported in the literature (see p. 23 to 24 at https://books.google.com/books?id=nxkHzjcjMe0C&pg=PA23&a... . However, the methods employs ethylene carbonate, a phosgene equivalent. The
reaction takes place at 70-100 C in the presence of chlorine (no solvent) and an initiator using a high-pressure Hg lamp. Interestingly, since
(CH2O)2CO is classified as the carbonate ester of ethylene glycol and carbonic acid, this prep is related to the previous procedure.
--------------------------------------------
Next idea, less toxic but much more speculative. Those acquainted with photolysis may remember how hydrogen and chlorine can be easily radicalized
with light at low temperatures. In the current case, we need to radicalize only some of the chlorine via photochlorination (see general reaction
mechanics outlined in Wikipedia at https://en.wikipedia.org/wiki/Photochlorination ). An important comment per Wikipedia, is, to quote:
“The selectivity of photochlorination (with regard to substitution of primary, secondary or tertiary hydrogens) can be controlled by the interaction
of the chlorine radical with the solvent, such as benzene, tert-butylbenzene or carbon disulfide.[14] The complex formation between benzene and the
chloride radical reduces its reactivity which increases the selectivity.[15] By varying the solvent the ratio of primary to secondary hydrogens can be
tailored to ratios between 1: 3 to 1: 31.[16] At higher temperatures, the reaction rates of primary, secondary and tertiary hydrogen atoms equallize.
Therefore, photochlorination is usually carried out at lower temperatures.[13)“
So, an attempt at photolysis would be to apply light in the presence of dry chlorine and dry oxalic acid in an appropriate solvent (per above or
none, just use less efficient gaseous nitrogen, since the net reaction implies only half of the Cl2 should be radicalized, I would try to avoid over
radicalization of the chlorine with a possible loss of yield). One of many speculated schemes includes:
Cl2 + hv → •Cl + •Cl
H2C2O4 + •Cl → HCl + •HC2O4
•HC2O4 + Cl2→ HCl + •C2O4Cl
•C2O4Cl + •Cl → (COCl)2 + O2 or, possibly unsuccessfully, 2 CO + Cl2 + O2
[ or, possibly as well:
•C2O4Cl + Cl2 → (COCl)2 + •Cl or, possibly unsuccessfully, 2 CO + Cl2 + •Cl ]
With an implied net:
2 Cl2 + H2C2O4 + hv → 2 HCl + O2 + (COCl)2
Note, my speculation on a key problematic reaction:
•HC2O4 + •Cl→ HCl + 2 CO2
Hence, my suspected need to limit the relative creation of •Cl to remaining Cl2.
-------------------
The literature also cites the use of atomic chlorine on aqueous H2C2O4 and FeCl3, which I claim is different from the dry acid (no H+ + C2O4(2-) ions,
see https://cdn-pubs.acs.org/doi/10.1021/ja01208a024 ) producing only CO2 and HCl.
[Edited on 27-8-2018 by AJKOER]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I strongly advise against the synthesis of dichloroacetylene. We had an incident a few months ago in my university lab when a
graduate student was a bit sloppy with a reaction, and dichloroacetylene was produced as a byproduct. It immediately exploded. Luckily, nobody was
hurt, since the reaction was on a small scale.
Anyway, intentionally trying to produce dichloracetylene is extremely dangerous. Reacting it with ozone would be sheer insanity.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Trichloroethene acts as a stabilizer for dichloroacetylene, but I am pretty sure it forms phosgene under ozonolysis or permanganate oxidation.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by AJKOER  | First, one may wish to investigate a claim of a successful photochlorination path to (COCl)2, which has been previously presented on SM, see http://www.sciencemadness.org/talk/viewthread.php?tid=8291 ..
------------------------------------------
So, an attempt at photolysis would be to apply light in the presence of dry chlorine and dry oxalic acid in an appropriate solvent (per above or
none, just use less efficient gaseous nitrogen, since the net reaction implies only half of the Cl2 should be radicalized, I would try to avoid over
radicalization of the chlorine with a possible loss of yield).
|
But chlorine decomposes oxalic acid to CO2 CO and HCl. Doesn't it?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
My prep avoids water as the targeted product (COCl)2 is also water sensitive.
Per this source: "A dictionary of chemistry and the allied branches of other sciences", Volume 4, by Henry Watts, page 250.
Link: http://books.google.com/books?pg=PA250&id=lYXPAAAAMAAJ#v...
"Chlorine does not act on dry oxalic acid; but in presence of water, decomposition quickly takes place, thus:
H2C2O4 + Cl2 = 2CO2 + 2HCl
A similar reaction is produced by bromine, hypochlorous acid, and the chlorides of easily reducible metals. Hence oxalic acid precipitates metallic
gold from auric chloride, especially on boiling;"
[Edited on 27-8-2018 by AJKOER]
|
|
|
boilingstone
Harmless

Posts: 8
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
| Quote: |
From what I've read about dichloroacetylene, I would have to strongly advise against its synthesis. According to wikipedia (again) dichloroacetylene
is extremely toxic, pyrophoric, and may detonate at its boiling point. It seems there is a good reason it was mentioned in "The War Gasses...". While
dibromoacetylene suffers from similar issues, apparently these issues are less severe. again, reference "The War Gasses..." I posted last time for
more info
|
| Quote: |
I strongly advise against the synthesis of dichloroacetylene. We had an incident a few months ago in my university lab when a graduate student was a
bit sloppy with a reaction, and dichloroacetylene was produced as a byproduct. It immediately exploded. Luckily, nobody was hurt, since the reaction
was on a small scale. Anyway, intentionally trying to produce dichloracetylene is extremely dangerous. Reacting it with ozone would be sheer insanity.
|
We seem to be in agreement. What then of dibromoacetylene? The acetylene halides become more stable as the halides proceed down the group.
|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
How about using oxamide? Displacement to chlorine seems plausible.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I would be afraid of generating cyanogen, which itself has a tendency to explode, polymerize or both.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
If someone makes oxalyl bromide, that would be interesting since it could be substituted in many places for oxalyl chloride, but there is much more
literature on oxalyl chloride. I would want to see a step that produces oxalyl chloride from oxalyl bromide.
|
|
|
boilingstone
Harmless

Posts: 8
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
JJay:
A quick search on prepchem shows a procedure for the opposite:
http://www.prepchem.com/synthesis-oxalyl-bromide/
|
|
|
| Pages:
1
2
3
4 |