| Pages:
1
2
3
4 |
golfpro
Banned
Posts: 179
Registered: 18-5-2013
Member Is Offline
Mood: Cap Sensitive
|
|
I hate ordering chemicals online but I might have to. I read about using ammonium nitrate for this and it won't work as well because of side reactions
as well as safety issues.
|
|
|
bfesser
|
Threads Merged
23-10-2013 at 18:23 |
bfesser
|
Threads Merged
23-10-2013 at 18:26 |
bfesser
|
Threads Merged
23-10-2013 at 18:38 |
bfesser
|
Threads Merged
23-10-2013 at 18:40 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Moderator Warning
golfpro, stop opening new topics to ask short questions. Use a search engine.
I've merged <em>nine separate topics</em> on HNO<sub>3</sub> from you! No more warnings; from now on, I will delete any new
topics you open.
|
|
|
cal
Hazard to Self
 
Posts: 88
Registered: 7-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by golfpro  | I hate ordering chemicals online but I might have to. I read about using ammonium nitrate for this and it won't work as well because of side reactions
as well as safety issues.
|
I made it frron sodiium nitrate and sulfuric acid in about 3 hours and distilled it with out any problems.
Thought is an action, which when acted upon becomes work and sometimes art!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I know this is an old post, but do you, or anyone have a reference for this?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I don't have one to hand but it is hardly needed as the method is easily understood ─ however difficult to practice?
Also, NO2/O2 fed to the azeotrope under pressure can attain up to ~98% HNO3!
Think I'll stick with the ~96% HNO3 I get from ordinary distillation!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
So would the equation be:
N2O5 + 2H2O → 2HNO3?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yep! All that's required now is a N2O5 generator and some water . . . ?
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Well never mind, looks like dinitrogen pentoxide is too expensive to be useful as a precursor to nitric acid.
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I read in an old book about distilling nitric acid using steel retort, and right now I want to build or improvised one because i want to make some
lead nitrate and I need diluted nitric acid.
What do you thing about this improvised distillery can work ?
I read on a forum about a "recipe" to make nitric acid without distillation but how pure can be the final product because i don`t want sulfates in my
nitrate.
| Quote: |
Nitric acid Reaction:
-Bring 100 mL of Distilled Water in a 500 mL pyrex beaker to 212F (100 C)
-Add the Nitre of your choice (202 gm K / 170 gm Na) -Stir until Nitre is completely dissolved, let cool below boiling
-SLOWLY add 56 mL concentrated (96%+) Sulfuric Acid to Hot Nitre solution while stirring. DON'T allow the solution to boil! -Allow solution to cool to
room temp (DO NOT SKIP - VESSEL WILL SHATTER IF PUT ON ICE WHILE HOT!!!)
-When vessel reaches room temp 77F (25 °C). Put the vessel in the freezer or on a salt water ice bath -Let stand until temperature of mixture reaches
-41F (-5 °C)
-Let stand at -41F (-5 °) until all precipitate settles
-Pour the COLD solution off into glass container with tightly sealed lid DO NOT POUR OFF ANY OF THE SALT IN THE BOTTOM!!! Makes ~160 mL ~ 50% HNO3
|
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
The process described is indeed distillation, it's just a simpler setup.
If done perfectly there should be very, very little sulfate in the distillate. If you use an excess of nitrate there should be even less.
But perfection isn't possible, and while very little sulfuric acid will volatilize, aerosols may be a major cause of contamination of not just
sulfuric acid, but also Na/KHSO4. Using boiling chips will alleviate the problem, but I can't think of a way to completely prevent
contamination.
BTW, your "beaker" doesn't look like it could handle the heat. Also if that's a plastic coke top, it will catch on fire, but that shouldn't be a big
problem right?
Actually I can see that it's glass, nice job cutting that, diamond blade?
[Edited on 11-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Bert
Super Administrator
        
Posts: 2822
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
About your quoted procedure- Just for a start: -41 F is approximately -41.5 C, NOT -5.1 C!
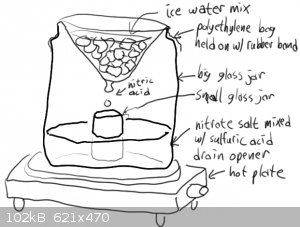
Your drawing may be workable- Particularly f you can can cool the small jar receiving the condensed nitric acid. Some have done what you diagram, but
added a ceramic or glass "stool" of some sort to keep the receiver above the hot nitrate/Sulfuric acid in the bottom of reaction vessel.
The second procedure in the below linked video starting @ 2:10, reacting a nitrate, Copper metal and hydrochloric acid while piping the nitrogen
dioxide into a hydrogen peroxide solution will give you useable nitric acid without sulfate contamination-
http://youtu.be/2yE7v4wkuZU
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
yes its been done BUT if the "big glass jar" is not heat resistant you may have a serious and i mean dangerously serious situation.surely you werent
gonna use that pickle jar looking thing?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Everyone can see that the text and the pictures otonel posted are two completely different processes right?
Both methods are dumb for different reasons, the one in the pictures is very dumb.
It might be preferable to make dilute nitric and distil than to make concentrated acid and dilute. If nothing else less toxic gasses should be
produced. Lead nitrate is a persistent poison. This sounds like a very green student wanting to do something stupid so he can do something much more
stupid.
|
|
|
Bert
Super Administrator
        
Posts: 2822
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Indeed, I missed the drawing not having a "bath", putting a non Pyrex container full of acid on a hot plate is asking for a disaster.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I understand how dangerous is lead nitrate and working with acids especially nitric acid, You have right about glass jar if it is not heat resistant
can make a disaster, that picture with the jar is not my "opera" and my mother when make pickle put the glass jar in a pot and warm the water for
better preservation, and the jar don`t have fissures after that.
In this weekend I want to try this distillation and I will tell about the result.
[Edited on 13-2-2015 by otonel]
|
|
|
Bert
Super Administrator
        
Posts: 2822
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Otonel-
Read up on heating "baths"-
http://www.sciencemadness.org/talk/viewthread.php?tid=19089
Water, brine, oil, sand, even metal shot.
If you still plan to put a glass jar directly on a hot plate, PLEASE try it once with nothing but water inside.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
Sorry for the misunderstanding, I will use pyrex container placed on a water bath sealed (not perfectly) with glass and heat resistant silicone .I do
not want a perfect seal because the heating increases the volume and a perfect seal can cause problems
I want to say a normal jar can be use if it is placed in a pot with water and this is slowly heated from a hot plate
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2209
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Canning jars are fairly resistant to thermal shock.
You really need an oil bath but I use them for various reactions
And haven't had one break. I wouldn't use direct heat on one though.
Safety first.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
negative gentlemen, stop this crazy idea about using this kind of glass.there are many shaped glass coffee vessels that can stand heat better than
soda lime glass.some coffee glass pots are even shaped as erlenmeyers and beakers and can withstand a lot more heat than ordinary glass.i have used
glass coffee pots on live fire and even propane flames,some have shattered upon cooling also.cease and desist now and be safe.
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
Improvised distiller at work in pics, it worked well with the condition to change top water at 5 minutes using a syringe.
Nitric acid obtained is concentrated and emits red smoke.
I am glad for successful experiment and it can be improved , especially the heat exchanger
  
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
very good otonel.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
What concentration did you get?
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I don`t determined the concentration because I diluted it anyway for use in what I need and do not emit toxic fumes.
After aspect I think is somewhere in the 70 % concentration
|
|
|
| Pages:
1
2
3
4 |