| Pages:
1
2
3
4
5
6
..
37 |
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
I am not very confident that the obtained product contains much phorone as it is non soluble in organic solvents, not yellow or green-yellow,
completely solid at temperatures even near 100°C and it only dissolves a little bit with orange to red colour in strong acids such as HCl or
H2SO4*6H2O, depending on quantity.
The powder is a very pale yellow to white one with the consistency of flour.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
conc. sulfuric acid with acetone
Polverone: If u want u can pass some of the articles to me for translation, i speak german.
25mL of conc. H2SO4 were carefully and slowly given to 75mL of acetone(heated up as with water).
Then the batch was heated at approx. 50°C for 2 or 3 hours till the liquid had again very dark colour.
In thin layers the main content of the flask turned out to be dark red.
Then the black liquid was diluted with double its volume of cold water and neutralized by addition of solid sodium carbonate.
I discovered that very big quantities of sodium carbonate are consumed and it is very time consuming to add the carbonate due to the vigorous
reaction.
On the other side, if using 25% ammonia, the neutralization affords less time and relatively low amounts of ammonia, maybe due to the fact that
ammonia is better soluble in the acetone-H2SO4 mix and thus enters more easily in the reaction.
Very much stirring and waiting has to be done if solid(solutions of the same would give too big volume) carbonate is used.
I´m not sure wether ammonia also reacts with the reaction products, but i tend to say that this would rather have a negligable effect if the ammonia
is not added in excess.
The most interesting thing about this all was the appearance of a small dark green, oily layer(very much like phorone could be, considering the temp
was approx. 20°C and the melting point of the product would be decreased by several byproducts) after dilution with water.
I seperated this by filtering(the oily substance was hydrophobic and did not pass the filter as it was always wettened by the aqueous solution running
through) and then i could seperate it from the rest of the solid product very easily cause it dissolved in both ethyl alcohol and acetone with orange
or black colour, depending on quantity.
I evaporated the acetone and got some mg of an orange to red-coloured non-crystalline layer on the bottom of the flask that smelled exactly like
incense.
After that i got a bit "stoned" breathing all those fumes over hours, i decided to come to an end and simply dissolved the small layer obtained with
about 20mL of HCl(half of it dissolved), put the black solution(notice the difference in colour to solution in acetone) in another flask and added an
equal amount of 30% hydrogen peroxide.
Immediately, from one second to the other, the black liquid cleared up and some white (crystalline?) substance swam on the bottom of the surface.
Now one would fear that such a substance could not have a good density, but considering the overall-density of the peroxidation-mixture, which is at
least 1,3g per mL, at least a crystal density equal to acetone peroxide would be possible.
Unfortuneately i had no time left to dry the white product and give it a try on burning "saltpeter-paper", but i will do this as soon as possible and
provide u with some photos of the batch.
HLR
(The board didnt let me enter as HoffmannLaRoche any more, so now i am BASF)
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
DBSP
I read the posts on E&W, but i can say that the inventor definitely didnt mean 9000ft/s instead of m/s!
It is a german invention!-A german would never use ft/s instead of m/s as the metric system is the the most widely used system here.
Furthermore, this would really represent a grave error that the patent office wouldnt tolerate in a patent.
The E&W-thread is not half as informative as the one established here, and is more or less a completely useless disscussion without any progress so
far.
HLR
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
After fiddling around with the structure of phorone which seemed very unfavorable to me, I let chemsketch calculate the 3D structure from the original
structure in the patent. Surprisingly, the 3D seems quite stable and relatively unstressed for an organic peroxide. The reason the 2D structure looks
so strange is that one cannot draw torsion angles in 2D structures which are a key variable to the stability of a molecule.
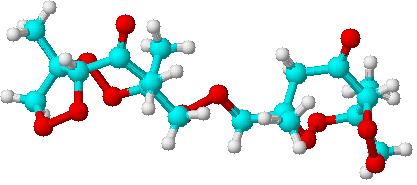
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Here is the same structure, only rotated around it's axis.
Chemsketch predicts a density of 1,3g/mL for DPPP.....
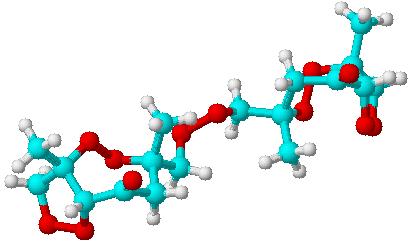
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
My god THATS good news.....1,0-1,3g/mL is the density of the peroxidation product i obtained recently using a product from one of my phorone
batches....this really seems to be a fascinating prog you were using.....never thought a simple prog for the home use could calculate densities...
HLR
|
|
|
ShockWave
Harmless

Posts: 7
Registered: 17-10-2002
Location: Netherlands
Member Is Offline
Mood: No Mood
|
|
Well I have tryed the procedure from Blast_X and it worked fine to me.
The Cristalls that I have are much smaller than AP, And it doesn't smell like AP, now I will let it stand to let the AP evaporate.
|
|
|
ShockWave
Harmless

Posts: 7
Registered: 17-10-2002
Location: Netherlands
Member Is Offline
Mood: No Mood
|
|
This is funny.
The Crystalls that I have standing now burns slower than AP, I heard that there is always some AP present in DPPP but AP blinks in light, like real
crystalls but my DPPP does not blink at all, It is also less white, and no smoke or smell after burning, the crytalls are very very small.
The wierd thing is that I did not do exactly what Blast_X told.
What I did was this:
10Ml acetone mixed with 10ml 30% Hcl, standing for about 10minutes, no color change just a clear liquid, than I added the 20Ml 30% H202 and kept the
temp. below 40c. after the first few ml h202 their was immidiatly a lot of with powder in the glass, When the 20ml h2o2 was added I stirred it very
well and waited for 1,5 hours, it looked just like AP, after that, I washed it very well with lot's of water and sodium bi carbonate, I let it dry for
1 day at a warm place, today I did these test and find it very intersting that this powder that I have is so very different compared to AP, If this is
pure DPPP, than it is just as easy to make as AP.
And that it burns much slower is an answer why the 5gr from Blast_X did not detonate but just burn.
5gr AP with a fuse ignition would detonate
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Sounds a lot like the results ( and procedure ) from my experiment. I also think that the fact that the product doesn't undergo DDT when ignited with
fuse, even if confined lightly, is a strong indication that it is not AP. However, my product sublimed relatively quickly...
|
|
|
ShockWave
Harmless

Posts: 7
Registered: 17-10-2002
Location: Netherlands
Member Is Offline
Mood: No Mood
|
|
Well, I just did a evaporate test, at a piece of paper I did a very small amount of AP and at the other end of the same paper I did some "DPPP", and
it was above my central heating which was not hot, but warm.
After 2 hours, the "dppp"totally evaporated, and the AP was evaporated just a little !
So the stuff that I have is not DPPP and not AP !
At the picture you can see the paper, to the left is the DPPP which is darker and has smaller crystalls, to the right is the AP, you can see it blinks
a little.

|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
wow! thats weird! did you press that stuff or do impact tests?
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Am I correct if i say that what's supposed to be DPPP is amorphous and the AP is crystalline?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
250ml Muriatic Acid in 250ml Acetone
Heat up no color change
Added the about 900ml of 3% H2O2 immediately (couldn't fin 30% at the momment)
Reaction (Acetone Peroxyde) precipitate at the bottom
Shaked realy well
The liquid turned yellow and Chlorine disgaged
All the precipitate went on the top and didn't sunk
I've let it stand 2 days then filtered
Did I get DPPP or AP?
And is it possible that AP converted in DPPP if Phorone formed during the shaking?
|
|
|
ShockWave
Harmless

Posts: 7
Registered: 17-10-2002
Location: Netherlands
Member Is Offline
Mood: No Mood
|
|
If you want to know if you have DPPP or AP, just do these tests that I did, the evaporate test works fine for me, And tell me, What color is your DPPP
and does it blink in bright light?
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
And is it possible that AP converted in DPPP if Phorone formed during the shaking?
No. The AP won't react with phorone to form DPPP, I don't know where you got it, but chemistry isn't mathematics (Thank God!). Take a look at an AP
structure and then at the DPPP structure in this thread.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Peroxidation of commercial phorone
I recently bought 97% phorone at Sigma-Aldrich, which seems to be the only supplier of small quantities.
At first, the phorone was very pure, could easily be melted in a warm water bath and crystallized upon cooling(room temperature) in the form of long
yellow needles.
Solubility tests:
-Insoluble in 30% HCl
-soluble in commercial acetone
-exzellent solubility in 98% acetic acid
-Insoluble in commercial ethanol(absolute ethanol needed?)
The phorone does not crystallize at room temperature if surrounded by an aqueous (immiscible) phase.
I tried to peroxidize the phorone(1ml) by first dissolving it in 98% acetic acid (5ml=excess to prevent seperation upon addition of the
peroxidation-mix)
and then adding a 1:1 mix of 30%H2O2 and 30%HCl(total of 2ml) in a test tube.
It heated up very strong and i repeated the whole procedure with proper cooling(ice bath), but so far no precipitate occured.
Maybe the DPPP is very soluble even in aqueous solutions of acetic acid, like phorone.
-Or the reaction just failed.
So i added some ml of water to make it seperate along with the rest of unreacted phorone, but nothing remarkable happened.
The last experiment i did was to dissolve phorone in acetone and then tried to peroxidize both.
I was surprised that the phorone seemed to prevent peroxidation of the acetone(no AP-precipitate).
HLR
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
I will try the evaportation test, and my powder is yellowish and don't blink
myabe i just got phorone?
|
|
|
ShockWave
Harmless

Posts: 7
Registered: 17-10-2002
Location: Netherlands
Member Is Offline
Mood: No Mood
|
|
Blind Angel
I think you have the same stuff as I have, If you have some AP left than do the evaporate test and see if if the DPPP evaporates faster.
Today I tested the "DPPP" but can not tell if it was stronger then AP, but the stuff detonated and if it is just AP than it is a nice procedure
because now you can make AP in less than 5 minutes with a great yield.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Do a little lead block test. Drill equal sized holes (with a volume of around 0.5cm3) in two blocks of lead. Fill one with CTAP, and one with "DPPP"
pressed under the same pressure, initiate both with a few mm of a primary such as lead azide or double salts (you want to be sure that none of either
explosive being tested is used up in DDT, so have a "fast" primary that has a short DDT time). If DPPP really is as good as the patent claims, which I
doubt, then there will be a significant difference in their effects. If DPPP is not as good as the patent claims, then it will be harder to tell if
that's what you have... you'd have to perform several identical tests and take the average expansions.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Nick, IIRC, the Trauzl test is not directly related with detonation velocity.
The sand test seems better to me then. Just use little rocks and see which are broken into the smallest pieces.
I could be wrong though.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
I putted some on a sheet of paper than light it up so i detonate. It didn't detonate it just did "Pshhhhh" and the flame got bigger but no "real"
explosion got detonated. I still have the yellowish solution from wich i filtrated the product, will try to freeze it (if phorone appears in cold)
then will try to heat it to see what happen.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
The VoD is certainly a factor that effects a substance's performance in the Trauzl test, along with energy content, density, gas volume produced, etc
(and all these factors have effects on each other, too).
E.g. ethylene glycol dinitrate gives a (water-tamped) standard lead block expansion of 650cm3, glyceryl trinitrate gives one of 590cm3 (COPAE). You
can see that EGDN produces a significantly larger expansion, even though GTN is denser by around 0.1g/cm3. This is largely because EGDN has a higher
VoD due to its lower vicosity.
But you should certainly be able to tell the difference between a 4km/sec VoD and a 9km/sec VoD(!), especially since the compounds are very similar
and are likely to have similar gas volumes.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Do u have further information on this test, myabe more graphical information I dont understand realy well how you can calculate the VoD
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
It is impossible to calculate the detonation velocity from the trauzl test, but it is possible to compare with other explosives whos vdets are known.
I´d propose making a lead block for 1 or 5g instead of 10g(its not that easy getting so much lead...and after using it, it is toxic waste).
More information on the trauzl test can be found in the cd-roempp-chemistry lexicon(100MB), Thieme Publishing, Germany.
I haven´t seen it on the net so far...but it is the most widely used chemlex here in europe in the schools.
Teachers often allow pupils to copy it.
HLR
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
I searched for Roempp, Rompp, Römpp but still cannot found a readable version (always find non-working .ica file) and more they're in german and i
cannot read german, do u have other interesting ressource on this 
|
|
|
| Pages:
1
2
3
4
5
6
..
37 |