| Pages:
1
..
33
34
35
36
37 |
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
(Originally written by Rosco Bodine)
| Quote: |
In a very real sense this thread is a " Classic "
It may serve a useful purpose to just leave the thread as it is , because it shows the contrast between scientific method and tests designed to
develop pertinent data versus intuition and anecdotal but non-conclusive approaches ,
applied to the task of learning an unknown by experiments intended to reveal what is secret . Reading through the thread , there are portraits of
various persons struggle with methods and tools for investigation , verification or dismissal of a set of reactions descrbed by an obscure patent .
The result is an "epic" work , which is sort of
the "War and Peace" of organic peroxide threads
|
Yep. There`s so much truth in that 
@vulture: it was great at the beginning, this kind of gold-digger-mood, was it? 
I want to repeat my offer on samples of my 97% phorone (from Sigma-Aldrich) ..... it could be used as reference for chromatographic comparison and the
like. - Since i dont have any ambitions in trying the peroxidation again.....U2U me, if interested....
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Gold digger mood is always fun in the beginning. But it quickly turns ugly when only pyrite is found and gold fever catches on.
Don't say I didn't warn you.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You've done the experiment , now see it all on DVD !
Yes , next it is being made into a feature length movie ,
" DPPP " ( part deux )
or perhaps .....
" Mackowiaks Folly " ( the sequel )  
coming soon to a theater of madness of the scientific variety near you ,
unless you spare yourself and escape now
while you still can .   
Better watch that boy ,
he's become anhydrously reactionary ,
singing
How dry I am ,
How dry I am ,
How wet I'll be ,
If I don't find .......
a place to pee.
[Edited on 22-3-2005 by Rosco Bodine]
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
Hello!
Dear Rosco Bodine,
As always thank you for your reply,
I didn't figure that patent was worth
much anyway. As I said in my latest
post to you I have enjoyed discussing
that blasting cap setup with you. I
didn't figure the scaling up of the idea
to a 2,000 pound bomb would have worked, or the military would have
discovered it along time ago. Well
here's our new place to talk if your not mad at me about what I said to "you know who" (name starts with an N) and how things are now
with "you know what" (name starts with R)!
As always, thanks for your reply!
P.S. By the way this is a very awesome
thread!
|
|
|
EBAYID_cheap_stuph
Harmless

Posts: 16
Registered: 26-3-2005
Member Is Offline
Mood: No Mood
|
|
dppp
I found the key. to get rid of the mesityl compounds you add very little h2o2 to the cold black liquid. and let it sit for a day in the freezer. the
black stuff will precipitate to the bottom. next add h2o2 to the yellow liquid and don't let the temp get over 5c. You will get dppp.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
It's not even funny any more. Give it up, Hideki.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Ok, here's the real secret. DPPP isn't going to be made by any reactions proposed in this thread.
However, we can make phorone peroxides. You may notice phorone is an alkene, it will react with chlorine to form phorone tetrachloride.
That can be peroxidized to phorone diperoxide. Since phorone is a ketone it will most likely also react at that point of the molecule to make
diphorone hexaperoxide.
All the reactions I just proposed are known reactions. Doing that will give you phorone diperoxide or diphorone hexaperoxide, I'm not sure if
the ketone part would react on such a large molecule.
Now, since everyone wants to make phorone, I found a preperation by searching.
http://67.15.145.24/~sciencem/talk/viewthread.php?tid=1203
So, there's the secret. I'm surprised nobody has mentioned it before (especially the alkene thing). Look over my proposed scheme, try and
find any flaws, but there are none. Alot for those of you were asking for reactions, they are here.
I will try this when I get some calcium chloride and a cheaper source of ammonia.
|
|
|
EBAYID_cheap_stuph
Harmless

Posts: 16
Registered: 26-3-2005
Member Is Offline
Mood: No Mood
|
|
If it is not dppp we are getting then why is it more powerful than AP? Do you think the process in the patent is BS but the product is real. If you
think about it there are explosives that are top secret. and maybe this is one of them.
-------------------------------
Yah, yah, yah. There are more daft patents than you think. Read the thread.
[Edited on 8-4-2005 by vulture]
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I did some paper chromatography of my H<sub>2</sub>SO<sub>4</sub> + Acetone mix, and found that it appears to be made up of at
least 3 or 4 different compounds.
I'll scan in the paper strips, I did the chromatography with isopropyl alcohol, toluene, acetone, methanol and water, to see both what the thick
black goo was made of, as well as what the different compounds were soluble in.
Only acetone could dissolve all the components, so it was fairly easy to seperate out one of the compounds by solubility alone.
Anyway, methanol seems to dissolve only the black coloured compound, leaving behind a pale yellow material.
I added some of the black goo to methanol, and filtered out a huge amount of dark green solid. Another wash with methanol got all the remaining black
chemical out, but I also had a large amount go through the filter and so I lost a large amount.
I then rinsed in water/methanol, and filtered again, losing even more of the green solid.
I dissolved what remained in acetone, and poured into 5 times the volume of water to precipitate it out. I filtered again, and as it was going
through the filter it slowly changed from the deep green colour to a sulfur yellow.
I ended up with maybe 5% of the original solid, so obviously I need to do something different, perhaps not use pure methanol as that seems to bring
things through my coffee filters.
Now, this stuff looks to me like the same colour of the first "DPPP" made in this thread. The mixture I seperated it out was far beyond the
oily stage, it's "oily layer" was actually a nearly solid mass. This could explain why I got so much of the stuff.
It also has a strong, unpleasant smell, but not strong enough to cause watery eyes like my "DPPP" could do.
It would appear I have found the yellow impurity.
It has a very high melting point, so it isn't phorone. After melting it solidifies to become a solid plastic mass.
I'll put up the chromatography strips later as well as some pictures so you can see the colour. I'll try to do better seperation of all the
components on the weekend.
If I get more hydrogen peroxide then I'll try to peroxidize the different chemicals, but that's no quarantee.
EDIT:
The paper strips:
http://img223.echo.cx/my.php?image=pc0016nz.jpg
Green Stuff (right after the first filtration):
http://img89.echo.cx/my.php?image=precipitate8qq.jpg
After it turned yellow:
http://img89.echo.cx/my.php?image=yellow5ug.jpg
Also, got another liter of 35% H<sub>2</sub>O<sub>2</sub>.
[Edited on 23-4-2005 by Chris The Great]
|
|
|
Pyro_boy
Harmless

Posts: 4
Registered: 26-4-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by responsible blaster
I have been in contact with a fellow by the name of The R_Sert on Rogue science and he gave me a link to this sites DPPP synth page.
I have produced some of the material in question, and the yeild is very good. It is also far more powerful than AP or APAN or MEKP. I think this
materials full power can be realized when it is highly compressed and blasting cap placed at dead center of the device. |
Interesting enough, I too made a batch of the material in question. I had to make it at very low temperatures and it is very similar to AP, but is
ever so slightly mint green/yellow. Crystals are cubic...
[Edited on 26-4-2005 by Pyro_boy]
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Holy Jesus Christ. If I attempted to read the whole thread, MEDGO. Yet somehow, you have managed to talk for 35 pages about a preparation of a
somewhat obscure organic peroxide and things related thereof. Shows how far humans are prepared to go in their quest to attain and perfect knowledge.
  
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
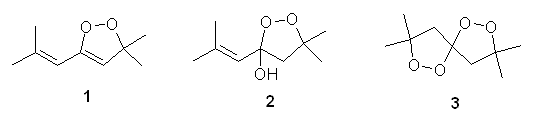
Excuse me, but I was just wondering how the formula of DPPP was obtained. I'm referring to the one that you all are referring to:
http://www.sciencemadness.org/talk/viewthread.php?action=att...
I tried to write some mechanisms.. and this is what showed to be the most obvious prods (hope I havn't posted same thing earlier in this thread
 ), see attachement. ), see attachement.
I'm too lazy to draw the whole mech.. but it doesn't include many steps..
It seems that prod 1 should dominate since reaction should start with H2O2 (hard nucleophile) attacking the carbonyl group (hard electrophile), rather
than attacking the conjugated C=C bond (soft electrophile).
Conversely, prods 2 and 3 starts with H2O2 attacking the conjugated C=C bond (prod 3 is formed from 2, although it could probably form from 1 too if
one of the double bonds was hydrolysed).
On the other hand, product 2 may also be dominating if we take into account that the carbonyl group is conjugated and therefore makes not that good
electrophile. While plus charge on the tertiary carbon is nicely stabilized (should be therefore in big concentration)..
Another good point to mention, prod 1 has an energy benefit due to the formed diene.. though it could be reactive..
Come on do anybody has a free access to a lab with a HPLC-MS instrument, where one could analyse the contents of that overly discussed reaction mix?
Some really soft ionization technique would probably reveal it all...
Without such data, going after smell/color of product seems to be quite useless..
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
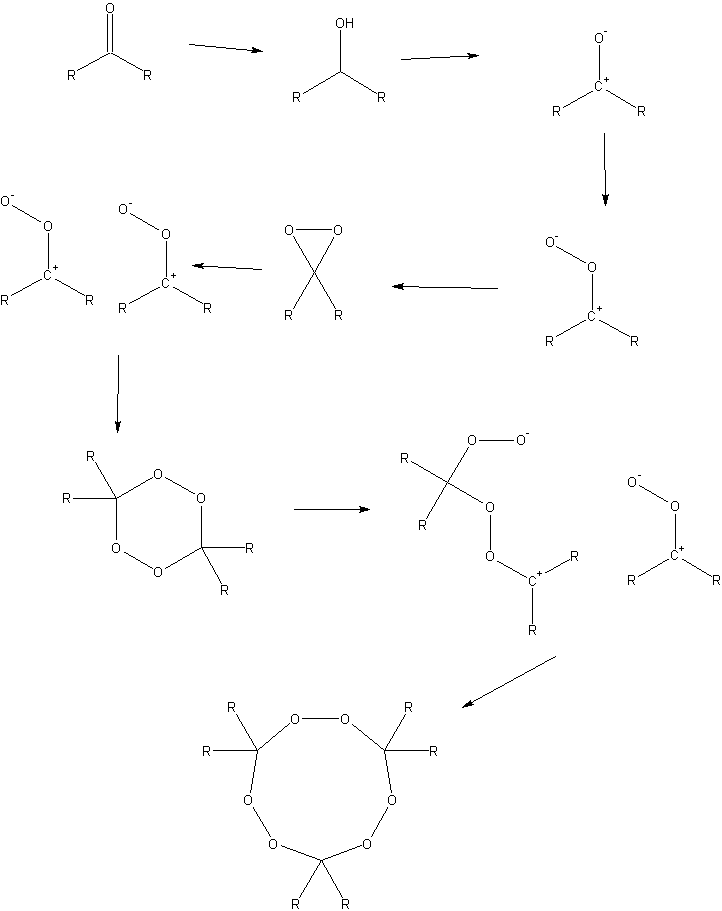
Peroxide formation scheme attached.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Frogfot, I thought the DPPP everyone was refering to was:
Attachment: DPPP molecule.pdf (18kB)
This file has been downloaded 1292 times
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
Oh, than I probably missed it in all those pages of this thread..
Though still, it seems that this dimer is quite improbable to form. You sure that this compound really is produced from phorone in one batch reaction?
I mean is there any other references than that german patent..
PainKilla, that mech wouldn't be true since phorone has some double bonds too..
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
No, that is the scheme for AP formation in my organic chem. textbook.... Im not saying its DPPP, but for general reference... We really have no idea
what the "real" molecule looks like anyway, so it could be like tetraAP or something.
|
|
|
Tweenk
Harmless

Posts: 15
Registered: 7-12-2004
Location: Poland
Member Is Offline
Mood: safety-consciou
|
|
Summary?
Could someone make conclusions of what has been done yet? ie some proven methods for preparation?
And now we add powdered sugar to the previously liquefied chlorine dioxide...
|
|
|
DirtyDan
Harmless

Posts: 40
Registered: 27-3-2004
Member Is Offline
Mood: Whizzed
|
|
Summary
In short, many claims have been made with many different syntheses and many different magnitudes of the compounds. Results have been black, green,
yellow, etc. Nobody is sure why and it could all be contaminents.
In other words, everyone is at the drawing board still (as far as I can tell)
Anyone have anything to add?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It's black and white .
DPPP doesn't withstand scrutiny .
|
|
|
Rusk
Harmless

Posts: 1
Registered: 24-7-2005
Member Is Offline
Mood: Tired
|
|
Hey, im a Yr 12 chem student, but i am quite advanced in contrast to most of my class, especially in the pyrotechnics field. I have been a member of
totse backyard ballistics forums for quite a while now, and i have taken a real interest in this "dppp" discussion. i have read through most
of your pages, and is far as i see it, i think this illusive "dppp" does not exist. Instead people have managed to create an alternative
form of AP, that is more stable, slightly more powerfull and is faster to create, without having to worry too much about trimer ap causing accidents.
I wander if what i have meantioned has summed it up, and if it has can someone post a full "walkthrough" to succesfully making this new
improved form of ap.
In Siberia, its too cold to cry
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
If you dump the chilled solution made from heating the acetone and HCl together into cold peroxide fast, then add household ammonia it makes an
awesome cleaning solution...I had spilled some of this a long time ago in a failed DPPP attempt and it cleaned the horribly stained bench very well.
I am posting this as I make more of this to clean some very badly chemically stained wood.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
So, the end product of this wild goose chase is... a cleaning solution. Perhaps the title should be changed appropriately? 
|
|
|
((Blasta))
Harmless

Posts: 29
Registered: 3-8-2005
Member Is Offline
Mood: No Mood
|
|
humm...going to wing this one.
When I was in high school I remember a demonstration using HCL gas in Acetone. The production Mesityl Oxide, Acetone DiAlcohol and Phorone all existed
in the same container. However, after about 2 months this liquid became resinous and globs of Phorone stuck to the pyrex flask.
On an interesting note Mesityl Oxide can certainly become a part of an unstable Organic peroxide...this much I know. Pure Mesityl Oxide is a dark
liquid which can easily react with Hydrogen Peroxide. However the temperature must be quite low or the heat generated by the two chemicals can cause a
SERIOUS and sometimes EXPLOSIVE run-away reaction. I think this is where people are having issues. The Patent "DPPP" is a fraud. I have
attemped this reaction several times in lab settings...each time I have created the same product. I produced my own Mesityl Oxide and I also used lab
grade Mesityl Oxide. In both sistuations I recieved the same product...this was a fine crystalline power which had similar properties to TCAP, but
appeared slightly yellow/green in color. This material was formed only at low temperatures, 0 Celcius to be exact.
use caution---explosives in transport
|
|
|
Dream of the iris
Harmless

Posts: 36
Registered: 26-9-2005
Location: Heart of the Sunrise
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Polverone
One thing that just came to mind: when I was young and foolish, I experimented with acetone peroxide, not really knowing what it was, and so did many
of my friends. One of these friends once claimed that he had made a batch of acetone peroxide using a huge amount of HCl. He claimed that the mixture
grew hot enough to boil, and that when it had calmed down he was left with crystals large enough to be easily visible to the naked eye, and that they
had a slight yellowish cast. He also claimed that, unlike other batches of acetone peroxide, this one would explode, unconfined, even in the smallest
amounts.
|
That happened to me, all though my AP didn't have a yellowish tint. The crystals were huge, but the solution didn't boil. All the AP fused
together to form a "chunk". One time my solution did boil; however, but I discarded the solution before any soild could form. I should
have pics of the crystals, unless I deleted them...
[Edited on 28-9-2005 by Dream of the iris]
|
|
|
coldzero
Harmless

Posts: 1
Registered: 29-9-2005
Member Is Offline
Mood: No Mood
|
|
lets test it
I have read this thread with alot of interest. And many people consider this to be another form of TCAP.
Nobody has tried to measure the density of the dpp product only to make comparisons againts color and texture. which mean nothing. Are we that bad in
chemistry that we cannot dry out a couple of grams of this and put it into a known volume of water and measure how much water it displaces?
|
|
|
| Pages:
1
..
33
34
35
36
37 |