| Pages:
1
..
30
31
32
33
34
..
37 |
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Joeychemist: You need to dilute the acid before starting anything unless you want an accident like Chris. I am sure you can add the acid but it would
need to be done very slowly, and you WILL experiance the heat of it sucking the water when you add H2O2. This may not be a bad thing though.
Mikael, so let me get this stright did you or did you not get a product from what you were making?
I think it was Rosco who mentioned that this was an intermidiate between HMTD and AP. I agree with this, but I am not sure yet what it is. I have a
feeling we shall know soon... 
----------How very interesting. I am came up with this during my bio midterm. It seems right, and even more right since it is known that acetone can
turn into isopropanol. Please don't flame me I am still learning organic chemistry and this is an educated guess. it could still be just AP. But
I am doubtful.
The acetone first polymerizaes, forming an alcohol, then continues to do so, until the H2O2 is introduced. The OH then forms a peroxide bond. I will
try doing the same procedure, substituing the acetone for isopropanol. Hmm.... Even more interestingly is that isopropanol is now used to make
acetone, so it is possible that we are getting a mix of this alcohol polymer, various oxides and mesitylene. It also seems much more liekly. Bromic
says he had a paper on ketone polymerization, Ill see how well it corresponds to this.
Also, I am not sure why isopropanol does not do this on its own, probably due to more reactivity from the acetone. Also, the presence of the all
strong acid/base seems to do something. Isopropanol boils at 85C, and that is about where I started to get some serious boiling. I really think this
should be investigated.

la]
[Edited on 31-1-2005 by PainKilla]
|
|
|
Mickhael
Hazard to Self
 
Posts: 65
Registered: 17-11-2004
Location: B.C. Canada
Member Is Offline
Mood: Terrificlawful
|
|
Yes I made a peroxide...
Yes I do appear to have made some type of liquid peroxide, whether it is the tiny clearish layer, or the larger pale red layer, OR that it is mixed
into the remaining yellow milky fluid is something I have yet to determine, I have a pretty tight work schedule so I will get to more tests as soon as
I can.
\"I shall not fear, fear is the mind killer...\"
|
|
|
responsible blaster
Harmless

Posts: 2
Registered: 31-1-2005
Member Is Offline
Mood: No Mood
|
|
Hello guys, another new person interested in your project.
I have been in contact with a fellow by the name of The R_Sert on Rogue science and he gave me a link to this sites DPPP synth page.
I have produced some of the material in question, and the yeild is very good. It is also far more powerful than AP or APAN or MEKP. I think this
materials full power can be realized when it is highly compressed and blasting cap placed at dead center of the device.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
dry weight of "mixed product" from residual peroxide doubled
The weight of the dried "mixed product" obtained from subsequent reaction of an additional quantity of acetone after the "DPPP"
reaction was complete , is double what the yield is obtained by only the reaction of the acetone contained in the "precursor" .
In a previous experiment a precursor made from 95 ml HCl and 100 ml acetone was treated with 40 ml additional HCl and then peroxidized with 280 ml 27%
H2O2 .
The yield was 98 grams .
The experiment was repeated with the same quantities and conditions , with a subsequent addition of an "extra" 100 ml portion of plain
acetone , 3 hours into the supposed reaction for DPPP . There was a massive increase for the yield of "mixed"
product produced by the reaction of the added 100 ml acetone with residual unreacted peroxide contained in the original mixture . The weight of the
thoroughly dried " mixed product " is 198 grams . A 100 % yield of ordinary trimeric acetone peroxide from 200 ml acetone would be 201.5
grams . There are different ways of interpreting the result depending upon what the product is believed to be , * If * the product is trimeric
acetone peroxide , then the yield represented by the 198 grams of product is 98.25% of the theoretical 201.5 grams .
So at the very least an extremely high yield process method has been devised for trimeric acetone peroxide . The product may be a mixture of trimeric
acetone peroxide with some other unidentified organic peroxide formed in the first three hours of the reaction .
But in either case , the weight of mixed product absolutely disproves the Mackowiak specified reaction route involving chlorination , where any
chlorine produced would consume the same hydrogen peroxide , as was found instead to be unreacted in the "DPPP" reaction mixture , and
utilized in subsequent reaction with additional acetone , to double the weight of "mixed product" .
The result of this carefully done experiment disputes the validity of the Mackowiak patent for the claimed compound " DPPP " .
[Edited on 31-1-2005 by Rosco Bodine]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Interesting.
A couple of questions: You added the acetone straight to the putatiive DPPP crystals, right? YOu didn't first remove the crystals, then use this
supernatant to add another 100 ml of acetone into? Because as such it may well be possible that the 'DPPP' is 'over-peroxidised',
meaning the DPPP could well react with the acetone to form a lesser peroxidised species. To find this out, you'd have to try addition of acetone
to the filtered CLEAR solution of supernatant, which is the leftover after the 'DPPP' reaction.
Another thing - having done plenty of experimental chemistry and such, it seems extremely unlikely that anyone would achieve such high yields, simply
because even IF a conversion is 99%, one'd lose at least a couple of percent during the purification, if not more (it sublimes, you have to
filter, dry it etc). If you claim that you miraculously have a purification loss of 1% I simply won't believe you  
Therefore I should think that the 'theoretical yield' does not apply here with respect to AP, simply because the molecular masses etc are
not correct to start off with. In other words, the final mixed product cannot be AP because yields such as this are impossible to achieve, and thus it
has to be a species that is DISTINCT from AP.
Anyway, let me know whether the supernatant can be peroxidised. That'd truly indicate that there is leftover H2O2 (even though that could be
easily determined anyway by adding MnO2 or something, after neutralisation).
[Edited on 31-1-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 206
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
Be extremely careful when mixing concentrated sulfuric acid with hydrogen peroxide in the presence of an organic material like acetone. Mixtures of
sulfuric acid and high-test peroxide (piranha bath) are used to clean things like chromatography columns, and MANY accidents have resulted because of
residual acetone catching fire/exploding.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by chemoleo
Interesting.
A couple of questions: You added the acetone straight to the putatiive DPPP crystals, right? |
correct | Quote: | | YOu didn't first remove the crystals, then use this supernatant to add another 100 ml of acetone into? |
No, because of the thixotropic nature of the slurry , separation would have involved manipulation losses , so the residual peroxide
from the DPPP synthesis was reacted in situ with the added portion of acetone . | Quote: | | Because as such it may well be possible that the 'DPPP' is 'over-peroxidised', meaning the DPPP could well react with the acetone
to form a lesser peroxidised species. |
Unlikely for any such amount of excess H2O2 to be somehow
"adsorbed" on the DPPP product , especially since if the DPPP product was DPPP formed by the chlorination route , there wouldn't be any
residual peroxide there to be "adsorbed" or to participate in some sort of "transperoxidation" scenario . | Quote: | | To find this out, you'd have to try addition of acetone to the filtered CLEAR solution of supernatant, which is the leftover after the
'DPPP' reaction. |
You can believe that if you want to , I don't . Testing for the presence
and activity of reactants contained in mixtures without separation is a common practice and only rarely produces skewed results from unlikely side
reaction scenarios as you suppose occurs . | Quote: |
Another thing - having done plenty of experimental chemistry and such, it seems extremely unlikely that anyone would achieve such high yields, simply
because even IF a conversion is 99%, one'd lose at least a couple of percent during the purification, if not more (it sublimes, you have to
filter, dry it etc). If you claim that you miraculously have a purification loss of 1% I simply won't believe you  
|
So I can't slip that one past you , okay . You are correct about the drying losses and when I said well
dried , I did not say anhydrous and recrystallized from toluene . Both samples were dried the same way to an apparent level of dryness based upon the
elimination of lumps and the material becoming a free flowing powder . Also there was a weight loss on drying "curve" observed where there
is a sudden decrease in the weight lost from water evaporation and the weight loss enters a low loss per hour where sublimation is predominating .
Technically , because of the concurrent sublimation and evaporation of the last few percent of water , there is no way to *completely* dry the
material without substantial sublimation losses . So for consistency , the drying and the yield calculations were performed the same way , so that
the amount of error for occluded moisture is about the same for each sample , and a fair comparison is achieved . If you wanted the absolute figure
for sublimed samples or recrystallized material rendered *absolutely* anhydrous , the aliquots which I have tested would put the actual yields for the
anydrous organic peroxide at closer to 85% for the isolated pure product . But then it is also true that
manipulation losses occur there also . So
to be precisely accurate is impossible without resorting to an actual chemical determination . Split the difference , the actual yield on a molar
basis for the anhydrous product is ~ 90 % . Anyway ,
the range of error will never reach an amount for any measuring adjustment sufficient to explain the "doubling" of the mixed product
comparison . | Quote: |
Therefore I should think that the 'theoretical yield' does not apply here with respect to AP, simply because the molecular masses etc are
not correct to start off with. |
My stoichiometry is valid and double checked . If it is wrong then you tell
me where and where exactly , in more words than just saying "the molecular masses are wrong" , because I use good numbers and I know what
I'm doing . | Quote: | | In other words, the final mixed product cannot be AP because yields such as this are impossible to achieve, and thus it has to be a species that is
DISTINCT from AP. |
I get yields in organic peroxide reactions always in the 80-90% range , sometimes a bit
higher , with the exception of course for tetrameric AP which runs about half that percentage . | Quote: |
Anyway, let me know whether the supernatant can be peroxidised. That'd truly indicate that there is leftover H2O2 (even though that could be
easily determined anyway by adding MnO2 or something, after neutralisation). |
Certainly it is the supernatant
which is the unreacted peroxide . And the first clue that made me aware of the unreacted peroxide was when I brushed a thermometer against my skin
and was actually given the "white burn" which skin receives from any substantial concentration of peroxide . The thermometer had been
removed from a supposed "spent" DPPP reaction mixture which should contain very little residual peroxide , and yet it was evident it was
still loaded with unreacted peroxide which should have been consumed in the chlorination reaction and subsequent peroxidation of the chlorinated
precursor to DPPP . Because there was still a lot of unreacted peroxide in the "clear liquid" of the spent mixture , this was when I first
began to doubt the entire matter of DPPP , and devised the "residual peroxide" experiment to confirm those well founded doubts . Anyone is
welcome to repeat the experiment and adjust the drying and yield calculations to suit their own satisfaction . The final conclusions will not be
affected , only the figures supporting the same conclusions will vary slightly from my own . But since the figures are comparative from one reaction
with the next , the errors introduced by method will cancel , so long as the same method is followed for each reaction being compared . I am
perfectly satisfied
with the accuracy of my conclusion regarding the lack of validity of the Mackowiak patent . The process does not follow the reaction course Mackowiak
describes , and the first clue you will observe for this resides in simply watching the thermometer and seeing that the thermal curve for the reaction
is not where it should be in relation to the quantities of peroxide having been introduced . The exotherm diminishes well before it should and the
temperature begins falling rapidly , when it should still be rising during the peroxidation . I have a lot of experience with syntheses and with
observing such clues , and honestly the thermal curve raised an eyebrow for me the first time I did this synthesis and every time thereafter . The
thermal curve simply never reconciled with the reactions described by the patent , not at any range of temperature where the reaction was run , and I
covered my bases there too , trying many different combinations to give the reaction every chance to work as advertised , if it was going to work ,
but it simply does not proceed in the way Mackowiak has declared .
|
|
|
responsible blaster
Harmless

Posts: 2
Registered: 31-1-2005
Member Is Offline
Mood: No Mood
|
|
Can u guys boil down what you are finding as it takes too long
I am finding that I am loosing my train of thought reading all the academic jargon. Please tell me weather or not anyone of you have actually tested
this material in an electronic velocity testing rig? Also to add you your academic material I beleive that it is the addtion of 1.5x more HCl during
the 1:1 synthesis and addition of heat to what we are currently doing that causes addition oxygen to bond with the phorone HCl from the 35% H2O2. My
opinion on the DPPP done so far is infact we have a low grade Phorone peroxide derivative and is not infact DPPP or DPHP.
I filter my so called "DPPP" out and the crystalline material is yellowish, I then run isopropylene alcohol through the crystals and let
them dry out completely, crystals are white-greenish tinted. I am find something interesting here, the more additional HCl added to the percusor
"phorone HCl" beyond the 1:1 ration outlined by the patent the more brisilliant the ending peroxide becomes. AM I ON TO SOMETHING HERE? or
shall I call it a fluke? 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Sublime any of the colored material and you will get white crystals and a colored residue which does not sublime . It may only be a trace , but it
will be left there , the impurity responsible for the color .
And Hideki , the only thing you are "on to" is playing with mommy's computer or maybe a friends , or the one at the library or cafe .
There's no magic to making DPPP since it isn't DPPP that you get anyway . How about some solubility and melting point data to tie things up
nice and tidy , or would that be overkill at this point ?
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
No I think it would be a satifactory conclusion to this thread. Unless of course you get MP's that are really wierd.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Sorry, but as no one answered my questions, I ask again: can I use 3% peroxide, and do I adjust the amount of HCL besides putting in more peroxide?
And, is there any conclusion as to whether there is ANY difference in explosive power between this and AP?
Thanks.
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I just happen to have on hand 4 liters of spectrophotometric grade toluene . That should give some good solubility data . I'll try to do the
solubility test tommorrow , unless the cold I am fighting has other plans for me .
Quince :
3% peroxide is insufficient concentration for getting any decent yields from peroxidation reactions . 40 volume hair bleaching peroxide , "clear
developer" sold at beauty supply shops is about 12% and is still not going to produce any good yields , but way better than the 3% . You are
much better off using 27% or 30% for
peroxidations .
As for the power , that remains an open question . The results of those tests done so far are promising but not conclusive , as there are variables
other than the chemical identity of the test material which could account for the few positive test results . It may be slightly more powerful than
AP made by usual methods , but not as powerful as HMTD in small diameters and quantities .
[Edited on 1-2-2005 by Rosco Bodine]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Mickhael, I noticed you are also in BC. Where did you get your peroxide?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Rona sells 35% in 1L bottles, that's where I get mine (I'm in BC too....we should have a blast off or something  ). ).
18 hours after the 14.5mL H2SO4 was added to the 55mL acetone, the mixture has thickened and become even darker. I took up 2.5mL of it with a
synringe and squirted it into my sink which was filled with water. The water turned black with stirring, and a slight amount of oil floated to the
top, though very little. However, some greenish gunk that is very sticky got stuck to the sink and the tip of my syringe which I used to stir it.
This stuff is sticky like glue, and sticks to everything. It is obviously a contaiminant, but by my estimate it maybe makes up only 5% by volume of
the mixture at most. I will atempt peroxidation tonight.
The HCl and acetone mixture is now a nice orange, which a redder layer near the top.
The NaHSO4 mixture has turned yellow, it appears to be going at the rate as the HCl, perhaps because the NaHSO4 had gotten wet before being added.
Now, I just had an idea. Some people bought phorone and tried to make DPPP from that, with no success. It MAY just work.
http://www.sciencemadness.org/talk/viewthread.php?tid=80
No more needs to be said. This could be a method to produce 'true' DPPP.
This process may also work starting with acetone and HCl, although I have no electrodes that would be able to do this, so I can't test it.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Evidently the myth of DPPP is going to die hard .
Never the less , I am inclined to kill it dead .
Have test tube , Will Travel 
Wire Rosco , Science Madness 
|
|
|
Del Rocco
Harmless

Posts: 43
Registered: 21-6-2004
Member Is Offline
Mood: No Mood
|
|
"Hello guys, another new person interested in your project."
Translation: "Hello guys, please don't notice that I'm back."
Responsible Blaster,
Has J-Scan's LAN extended a bit or are you simply the next incarnation of Matsumoto Hideki/Mr. Anfo/J-Scan? Sure, your tune has changed minutely
but I cannot help but notice the obvious (one might say I have a rather keen eye for it):
1. Your first post you overcompensate your guilty feelings for coming back yet again where you are obviously not welcome by immediately offering
explanation of how you supposedly got here.
"I have been in contact with a fellow by the name of The R_Sert on Rogue science and he gave me a link to this sites DPPP synth page." (RS
has been inaccessable for at least the past 24 hours.)
2. "It is also far more powerful than AP or APAN or MEKP." AND "I am find something interesting here, the more additional HCl added to
the percusor "phorone HCl" beyond the 1:1 ration outlined by the patent the more brisilliant the ending peroxide becomes."
Still defending it to the bitter end are you, Hideki? Why does this patent require our attentions so badly? Did your daddy write it? (BTW, the word is
"brisant" not "brisilliant". I know this because I know an expert in-- "Word-iology". 
3. "Please tell me whether or not anyone of you have actually tested this material in an electronic velocity testing rig?" (Does anyone
really need to GUESS where he's going with this one next? It HAS to be at the very least 9000 MPS!)
I'll shut up, now, and take my lashings like a man if I am wrong, but there is only one way to tell... A mod needs to check his IP against that
of his other incarnates, that's all.
[Edited on 1/2/2005 by Del Rocco]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Toluene solubility comparison test results
In two 100 ml volumetric flasks was placed carefully weighed 50 ml of toluene
and a micro stirbar was placed into each .
The solubility of trimeric AP was found to be 18 grams in the 50 ml toluene . A small
amount of water separated and appeared
as tiny scattered droplets adhering to the walls of the flask . The amount of water was very small , insufficient to actually coalesce into a single
drop or settle to the bottom of the flask , just a few barely visible specks of free water , indicating the sample of AP was very dry .
The solubility of the " DPPP " was also 18 grams in the 50 ml of toluene . However ,
there was a huge amount of trapped water , slightly more than one third the weight of the apparently "dry" crystals of DPPP was entrapped
water , which separated when the DPPP crystals were dissolved in toluene , and formed a distinct lower layer of separated water .
The separated water was measured and subtracted from the weight of
" DPPP "which had been dissolved in the toluene to determine the non-water portion of the "DPPP" sample which dissolved as 18
grams .
These 18 gram per 50 ml solubilities are actually slightly into the cloudy range , a bit oversaturated and showing a tiny amount of undissolved
"grit" of crystals , so the "formal solubility" may be a fraction of a gram under 18 grams per 50 ml , or based upon the more
usual 100 ml of solvent , a bit less than 36 grams per 100 ml toluene . That's as close as I am going to get it . Anybody wants a more precise
figure can do the test themselves using recrystallized anhydrous samples .
Seeing how much occluded moisture persists in the " DPPP " has pretty well shitcanned any further interest for me in this material because
there's too damn much water trapped in the stuff . This makes for tedious work recrystallizing to get rid of the water . And the solubility
being identical to trimeric AP pretty well
says that "DPPP" *is* trimeric AP any damn way , which can be easily made in a much more pure and *dry* form without any elaborate
purification required .
I think any further interest for me with experiments concerning " DPPP " is finished after this toluene solubility test .
For me " DPPP " is going into the file
entitled " No-such Pentaperoxide " along with a few other other lame patents .
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Well then, thats that  . I guess everyone should thank each other (and especailly
rosco) for spending thier time and hard work in pursuit of this topic. 3 Years now is it? And the validity of DPPP is disproved! I guess I will use
this method since I can store the red/yellow much for a long time and make the peroxide whenever. I guess we can conlude its AP, but a
"better" form of it, which is not a bad thing. . I guess everyone should thank each other (and especailly
rosco) for spending thier time and hard work in pursuit of this topic. 3 Years now is it? And the validity of DPPP is disproved! I guess I will use
this method since I can store the red/yellow much for a long time and make the peroxide whenever. I guess we can conlude its AP, but a
"better" form of it, which is not a bad thing.
I thank everyone for spending their time on making this! I guess now we can disprove IPA Peroxide now  . I bet you it COULD work via that reaction mechanism I posted. . I bet you it COULD work via that reaction mechanism I posted.
O yea, I think using the H2SO4 helps prove that this is indeed AP since we know AP is also made via H2SO4 and that no chlorination is indeed
involved.
O yea, BYE HIDEKI!!!!
[Edited on 1-2-2005 by PainKilla]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Serendipity one more time
The good solubility of AP in toluene at room temperature is likely even better when the solvent is warm . And the solubility of AP in alcohol is
about one tenth the solubility in toluene . So this provides a possibility for securing the AP in anhydrous form pretty easily .
If the AP still damp from neutralization and filtering was dissolved in warm toluene , then any water present would simply separate from the toluene
solution of AP . If alcohol was slowly added to the separated toluene solution of AP , the AP should crystallize out in anhydrous form , and being
wetted only with anhydrous solvent which should evaporate quickly from the filtered crystals , which would be obtained water free . This could
certainly expedite the process of obtaining AP in pure and dry form . The AP contaminated
filtered solution of toluene alcohol need not go to waste , as it would make a very good solvent-fuel / detonation catalyst for wetting AN ,
especially if any nitro compounds
such as picric acid , or dinitrotoluene , or nitronaphthalene were added to the AP/toluene/alcohol , and then the AN dampened with such a mixture .
The anhydrous AP crystals could be used for the detonator / booster and any leftover AP simply blended into the sensitized AN mixture . Waste not
want not , and no leftover material to cause any unsightly clutter in storage areas 
[Edited on 2-2-2005 by Rosco Bodine]
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
So, we're going to ignore the different crystal shapes, the fact that it doesn't small like AP, and the fact that it, at least according to
those who have made it, that it is more powerful?
I mean, the stuff sure doesn't seem to be AP to me. I mean, sure the patent is crap, but this definatly isn't AP. Sure, it might not be
superpowerful ultimate explosive, but it seems to be something new.
There are simply to many open ended questions just to say it's AP.
Now, if someone does and NMR or mass analysis on this stuff, and it turns out that yes it IS AP, then ok, I'll believe it. No such test has been
done and it seems to have so many differences to be AP. If the crystals where the same, then I'd believe them. But the crystals are different!
I've done alot of close looking at my AP, and ever description of 'DPPP' I've read says, in big bold letters "NOT AP."
Since I mixed up some of my own, I'll test it myself, scent wise, crystal shapes and explosive power, and tell you. The method of formation
looks different, the smell is different, etc.
Not to seem like I am thinking like Hideki, but this stuff really seems to be a new peroxide, definatly not 9km/s or even close, but more brisant than
AP. And with rosco finding it so full of water, it might be more powerful if the time is taken to recrystalize and dry the stuff.
I am certainly not going to give up this stuff. I want to know what it is! And even if it isn't DPPP, I think we should at least preserve the
name as this whole thing really is an epic project.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
How about a mixed product recrystallization from toluene
Well that's next on my agenda . I have 18 grams of AP dissolved in 50 ml of toluene and I have 18 grams of " DPPP " dissolved in
another 50 ml of toluene .
If I mix the two toluene solutions , and start adding alcohol , or just dump the solutions out in a glass tray and let the toluene evaporate , either
way I'll bet anything only one variety of crystals is obtained , lovely snow white crystals of AP , and no crystals of anything else .
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Yes, that may convince me, although I will want to confirm it myself, after all I have a small batch of 'DPPP' in the works, and wow, this
stuff sure gets full of crystals! One of my mixes is nearly solid! Details below, as details are good.
My HCl and acetone mixture, which had been sitting for approximatly 3 days, and was a light red orange colour, was put into a hot water bath for about
1 hour, with about 10 minutes out of the bath because it started boiling without enough room to condense. Half was poured out and the jar was
resealed with a plastic baggy (polyethylene). Heating continued until the mixture was a black colour, although exposure to very strong light showed
it was a red colour. A small amount of oil was seen floating on the surface.
http://img41.exs.cx/my.php?loc=img41&image=heating2nl.jp...
This was put into the freezer along with my H2SO4 mixture and the ruby red HCl mixture I poured out 20 minutes in, as well as my H2O2 to get it nice
and cold.
12mL of the red stuff was put into a 125mL jar in a saltwater bath (-10 degrees).
http://img41.exs.cx/my.php?loc=img41&image=step19tf.jpg
Picture mainly to show contrast to later images.
10mL of H2O2 (35%) was added over a peroid of about half a minute with another syringe, with swirling. The temperature rose to 10 degrees. The jar
was capped and labelled A.
A second 125mL jar was put into the salt water bath, and 12mL of the red stuff was added, followed by 1 mL muriatic acid. 10mL of H2O2 (35%) was
added over a peroid of half a minute with swirling. Temperature was about 12 degrees afterwards, however the precursors had warmed up somewhat before
hand.
30 seconds later:
http://img41.exs.cx/my.php?loc=img41&image=30sec3aa.jpg
Maybe 2 minutes later:
http://img41.exs.cx/my.php?loc=img41&image=mins8ds.jpg
A fair amount of crystals had already formed at this point.
About 2 hours after this, the mixtures are both white. A is a near solid mass of crystals, while B is much more runny. Both smell nothing like AP,
and have a strong smell that burns my nose if I inhale right over the jar. I'm going to leave it for at least 12 hours before filtering and
washing, rinsing with alcohol and drying.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Can standard AP and the new version be used to detonate nitroglycerin/nitrocellulose gel? Should it be loose crystals or compressed?
Also, will it still detonate if I mix in a bit of polystyrene dissolved in acetone to bind it together?
[Edited on 3-2-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
Look what Mega has found, it is not that important but it still merrits a post
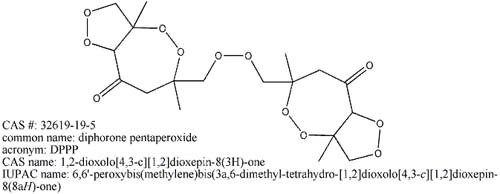
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Now that does definatly merit a post. 
However, the properties of the "DPPP" that has been prepared in this thread tend towards it being standard CTAP. Looks like we are in for
another 30+ pages added on to this thread in the quest for the actual DPPP. Unless what we have really is DPPP which seems quite doubtfull at the present time. I really would like to see NMR or mass spec done on
"DPPP" and CTAP.
Unless what we have really is DPPP which seems quite doubtfull at the present time. I really would like to see NMR or mass spec done on
"DPPP" and CTAP.
[Edited on 6-2-2005 by rogue chemist]
|
|
|
| Pages:
1
..
30
31
32
33
34
..
37 |