| Pages:
1
2
3 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Lead adducts of polynitro aromatic acids
Regarding the interesting patent that Axt attached above:
What is perhaps interesting that these lead salts aren't obvious acid base products, but adducts of some sort, and that they involve the nitrogroups
themselves.
They are formed by dissolving the polynitroaromatic acid in excess of PbO, or by dissolving the lead salt (as formed by precipitating with PbAc2) in
excess PbO.
Using *less* than i.e. 4 moles of PbO with lead picrate, however, does not yield the desired (precipitated) product, so it does need this 4x excess.
It is however possible to add 1 mol of Pb to each nitro group if a PbO (6 molar excess rather than 4) is stirred into *hot* solution of lead pictrate.
The reaction takes some time and its completion is indicated in all cases by precipitation of the adduct.
They tested this for (where the starting compound was the ordinary lead salt in all cases)
- Lead picrate, forming an adduct containing C12H4O18N6Pb5 (4 mole PbO to 1 Pb picrate) - dark yellow and insoluble
- Lead trinitroresorcinate (red powder, deflagration at 255 deg C), containing C6HO11N3Pb4 (3mole PbO to 1 PbTNR)
- Lead trinitrobenzoic acid (red powder), C14H4O20N6Pb5 (4 mole PbO to 1 PbTNBA)
- Lead nitranilate (red powder) (2 mole PbO to 1 PbNA) C6O10N2Pb3
I wonder what the structures of these things are, for instance the adduct of the PbTNBA...
All these adducts are different to the normal lead salts because of
- higher insolubility than normal lead salt
- higher sensitivity, i.e. the Pb picrate adduct explodes when exposed to flame while normal Pb picrate doesn't.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
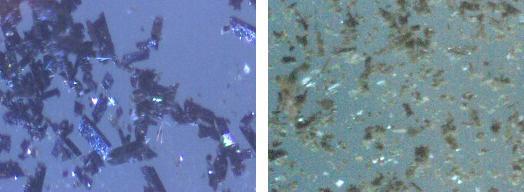
I left some of the tetraazide on a slide under the microscope, the decay after 40 days can be seen in the attached picture.
Also KNO3 + HCl can be used just as effectively as HNO3 + HCl for the production of chloranil from paracetamol. The reaction runs much the same though
is less vigourous in the initial stages. The crude yield from 12g paracetamol, 65g KNO3 and 150ml 32% HCl was 12.8g on the first try and 13.4g on the
second, effectively the same. For simplicity the first try used the whole tablet including gelatine capsule with the presumption that chloranil would
be the only product to resist the aqua regia.
|
|
|
YeOldeImpurities
Harmless

Posts: 8
Registered: 23-12-2007
Location: Europe
Member Is Offline
Mood: D'oh!
|
|
I tried NH4NO3+HCl, also works fine.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by Axt
I left some of the tetraazide on a slide under the microscope, the decay after 40 days can be seen in the attached picture.
|
Axt,
Maybe good to test if it is really a decay or simply sublimation.
By making the test in a tiny hermetically closed vessel.
Overpressure in the vessel will confirm the decay.Large cristal growth on the detriment of tiny ones would mean sublimation. Or maybe both processes
are in action.
| Quote: | Originally posted by Rosco
We need some Belgian input here
Where's Philou Zrealone when you need him ?
Need a nice clean dioxin free chloranil synthesis ,
from OTC materials so how about it ?
|
 paradichlorobenzene is a good cheap OTC source as mentionned hereabove. I have
like 10kg or so bought as toilet desodouriser (maybe 5$ the kg). paradichlorobenzene is a good cheap OTC source as mentionned hereabove. I have
like 10kg or so bought as toilet desodouriser (maybe 5$ the kg).
What is super interesting with chloranil, it the ease with what it is done an the exchange ability of its chlorine atoms.
[Edited on 24-1-2008 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That's great , finally having a way to make use of the
chlorinated variant of moth crystals .
That is something which was being discussed in Axts pentryl thread , but nitration seemed to be undesirable
as way of making the paradichlorobenzene into another
more reactive ring compound . So converting the moth crystals to chloranil would be much easier , and then finding possible usefulness for the
chloranil as an intermediate would be more practical .
So .......what do you think about the possible reaction
of chloranil with sodium tetrazolylazide , do you think
the theoretical compound I proposed would result in
2,3,5,6-tetra-tetrazolylazidobenzoquinone ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by Rosco Bodine
So .......what do you think about the possible reaction
of chloranil with sodium tetrazolylazide , do you think
the theoretical compound I proposed would result in
2,3,5,6-tetra-tetrazolylazidobenzoquinone ? |
The answer must be yes  when you see how easilly it fixes NO2(-) and N3(-). when you see how easilly it fixes NO2(-) and N3(-).
Although with chemsketch, you get a very bulky starshiplike structure...density is very hard to predict.
Maybe by plotting cyanuric triazide with cyanuric tritetraazolylazide,vs tetraazidoquinone it might give a density hint for
tetratetraazolylazidoquinone (TTAAQ).
On another aspect nitranilic acid makes a very beautiful planar molecule with H bondings or with the keto oxygen or with the nitro next to it...making
pseudo polycycloaromatics structures...Resonance of the pheno-keto-nitro groups allow transient passage to a triketo and tetraketo ring apparented to
the hexacarbonyl ring...the proton or sodium atom then jumping from the phenol group to the vicinal nitro group turned into a nitronic acid...this
might explain the sensitivity of nitranilates.
I suspect the tetranitro compound can be formed in anhydrous media...but like it is the case with hexanitrobenzene turning into trinitrophloroglucidol
(1.3.5 trihydroxy-2.4.6 trinitrobenzene), you have one of the two vicinal nitro that is subject to nitro-nitrito rearrangement and subsequent fast
hydrolysis.This effect is enhanced by the keto group.
Would also be interesting to test for tetracyanocompound by substituing the chloroatoms with NaCN or AgCN...this should be an energetic fuel...most
organic cyanides do have a lot of energy to give...see the heat of combustion of cyanogen (C2N2) vs acetylene vs ethylene vs ethane...you can reach
>4000°C in the flame.
Actually also sodium nitroformiate, sodium dinitramide, disodium dinitrourea would be woth a try  
[Edited on 25-1-2008 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by PHILOU Zrealone
Axt,
Maybe good to test if it is really a decay or simply sublimation. |
The decomposition at room temperature is well documented in the literature, I just provided the visual reference to it. The crystals changed colour
and jumped around the place like "fleas" to use a recent analogy from another member, for a different compound.
The reaction of chloranil is immediate with bases, both NaOH and Na2CO3 immediately turn the solution dark purple, the product is soluble in water. If
done in H2O2 solution the initial dark purple would turn clear if left stand.
I tried to form a peroxidic compound from cloranil by treating a slurry in methanol with NaOH + H2O2 solution. Again it turned dark purple but a tan
coloured solid remained, this was filtered. On ignition it smouldered, though every now and then it would POP! So something energetic was in there,
somewhere. A pure compund was never expected by this route though it may be possible to increase the "active oxygen" to the point where an explosive
is produced.
I did this as a simular method has been used to produce peroxides from cyanuric trichloride.
[Edited on 8-2-2008 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
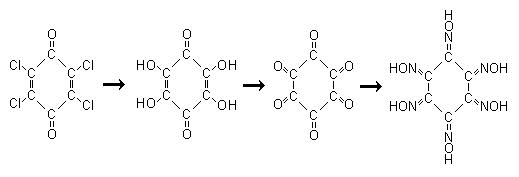
Since the topics turned into any energetic chloranil derivative, heres an idea to produce the hexaoxime which should be an interesting precursor to
other energetics. Or used alone as its salts being empirically similar to the fulminates, structurely different but I've previously shown the Pb and
ag salts of glyoxime to be quite energetic. Theres also possible furazan and furoxan (eg. BTF) derivatives from NaOH and NO2 respectively.
So its all OTC paracetamol -> chloranil -> tetrahydroxyquinone -> cyclohexanhexone -> cyclohexane hexaoxime.
Putative chloranil has been worked out in this thread.
Tetrahydroxyquinone even though it seems obvious it cannot be produced from NaOH in water, at least not easily. This is from J. Chem. Soc., Abstr.
60, 1027-8 1891.
<i>"Chloranilic acid is obtained when chloranil (10 grams) is moistened with alcohol and added to a solution of sodium hydroxide (9 grams) in
water (200-220 c.c.) heated to 80°, or to a solution of potassium hydroxide (12 grams) in water (250 c.c.). After remaining 1-2 hours, common salt
(20 grams) is added, and the precipitated sodium derivative is washed with a 10 per cent. solution of common salt until the filtrate is colourless; it
is then redissolved in water, and the chloranilic acid precipitated with hydrochloric acid. The yield is 62 per cent. of the weight of the
chloranil."</i>
So it will only replace two of the chlorines before the others are deactivated. Though a way around this seems to be found in Zeitschrift fuer
Naturforschung, Teil B: Anorganische Chemie, Organische Chemie (1978), 33B(10), 1201-3. This is the abstract, I'll try source the full article.
<i>"A simple synthesis of 1,4-tetrahydroxybenzoquinone.
Tetrahydroxy-p-benzoquinone (I; R = H) was prepd. by methoxylation-hydrolysis of chloranil and converted into various ethers (I; R = C2-C5 alkyl,
EtOCH2CH2) by treatment with the alc. in the presence of NaOH."</i>
So it seems the alcohol derivative is first formed and hydrolised to tetrahydroxybenzoquinone. Hopefully its as simple as reacting it with a hydroxide
in ethanol. There is another preparation in Organic Syntheses; Vol. V, p. 1011 though its an awful waste on glyoxal.
Cyclohexanhexone is formed as its octahydrate by oxidation of the sodium salt of tetrahydroxybenzoquinone with 25% HNO3, the simple preparation is in
J. Org. Chem.; 1986; 51(26); 5241-5243. Its just a mix, let react, cool, filter reaction.
Cyclohexane hexaoxime is an unknown, presumably it will form from Cyclohexanhexone and hydroxylamine though there is no references to it. Even
scifinder gives nothing except a few suppliers for it in milligram quantities, so it is at least a known compound.
[Edited on 20-5-2008 by Axt]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Food for thought on precursors
* N O T E
It may be necessary to use the following proceedure to extract the files
http://www.sciencemadness.org/talk/viewthread.php?tid=8997&a...
.
[Edited on 20-5-2008 by franklyn]
Attachment: C6O6 derivations.zip (1.3MB)
This file has been downloaded 710 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Triquinoyl ( cyclohexanehexone )
References
J. Lerch, Ann. 124, 34 (1862)
R. Nietzki and T. Benckiser, Ber. 18, 499 (1885)
R. Nietzki and T. Benckiser, Ber. 18, 1833 (1885)
R. Nietzki and F. Kehrman, Ber. 20, 322 (1887)
F. Henle, Ann. 350, 330 (1906)
F. Bergel, Ber. 62, 490 (1929)
B. Eistert and G. Bock, Angew. Chem. 70, 595 (1958)
B. Eistert, G. Bock, E. Kosch, and F. Spalink, Chem. Ber. 93, 1451 (1960)
Triquinoyl octahydrate C6O6 8H2O
forms microscopic needles, melting at 95º C, evolving CO2.
Insoluble in cold water, alchohol and ether. Is hydrolysed into
hexahydroxybenzene in acidic solutions with lewis acid catalyst
of SnCl2
.
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I couldnt find any reference to the reaction of hydroxylamine with triquinoyl so I looked at hydrazine to see if it at least reacted like a normal
ketone thus forming a hydrazone, however it does not. Rather its reduced to hexahydroxybenzene. Well at least I gather thats what the following
abstract is saying. I think where they said "cyclohexenone" they meant "cyclohexanehexone", the last entry.
<b>Reaction of organic compounds with hydrazine. XI. Reaction of quinone compounds with hydrazine hydrate.</b> Akita, Tadashi.
Univ. Tokushima, Yakugaku Zasshi (1962), 82 91-5. CODEN: YKKZAJ ISSN: 0031-6903. Journal language unavailable. CAN 57:48998 AN
1962:448998 CAPLUS
Abstract
cf. Tokushima Daigaku Yakugaku Kenkyu Nempo 9, 11(1960); CA 55, 19925e. p-Benzoquinone (1.1 g. ) in 20 ml. EtOH and 2.5 g. 80% N2H4.H2O heated 30
min. on a H2O bath, the EtOH removed, the residue acidified with HCl, and the product extd. with Et2O gave 1 g. hydroquinone, m. 172° (EtOH). The
same result was obtained by using 20 ml. diethylene glycol as solvent and heating 2 hrs. at 180-200°. Similarly, redns. of the following compds.
with N2H4 were carried out (starting material, product, % yield, and m.p. given): o-benzoquinone, pyrocatechol, 81, 105°; 1,2-naphthoquinone,
1,2-(HO)2C10H6, 80, 103-4°; 1,4-naphthoquinone, 1,4-(HO)2C10H6, 79, 190-1°; 1-methylp-benzoquinone, methylhydroquinone, 80, 124-5°;
hydroxy-p-benzoquinone, 1,2,4-C6H3(OH)3, 83, 140°; nitrop-benzoquinone, nitrohyaroquinone, 62, 133-4°; bromop-benzoquinone, bromohydroquinone, 66,
113-15°; 2,3-dicyano-p-benzoquinone, 3 ,6 ,1,2-(HO)2C6H2(CN)2 , 75, 230° (decompn.); chloro-p-benzoquinone, chlorohydroquinone, 60, 106°;
2,6-dichloro-p-benzoquinone, 2,6,1,4-Cl2C6H2(OH)2, 77, 157-8°; 2,5-dichloro-p-benzoquinone, 2,5,1,4-Cl2C6H2(OH)2, 77, 172°;
2,3-dichloro-p-benzoquinone, 2,3,1,4-Cl2C6H2(OH)2, 77, 144-5°; chloranil, tetrachlorohydroquinone, 60, 232°
2,5-dihydroxy-3,6-dichloro-pbenzoquinone, 3,6-dichloro-1,2,4,5-benzenetetrol, 66, --; cyclohexenone, benzenehexol, 88, above 300°.
|
|
|
SilencePlease...
Harmless

Posts: 5
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
Sorry to be such a bother, but as I'm devoid of a fume cupboard (chloropicrin) I was pondering over other methods of chloranil, preferably those with
decent yields.
As described by the "CIA Field Manule"; ASA + HCl + KClO3.
I was thinking about the use of p-acetylaminophenol + HCl + MnO2/Al
Would NaOCl suffice? I know it chlorinates other ketone, but looking at this mechanism, it seems unlikely.
Am I being over-paranoid? Should I just allow it (NO2Cl method) to take place at the bottom of my garden on a windy day?
Many thanks, SP...
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by SilencePlease...
Am I being over-paranoid? Should I just allow it (NO2Cl method) to take place at the bottom of my garden on a windy day?
... |
I couldn't detect any chloropicrin being formed when aqua regia (its NOCl) was acted on paracetamol, in fact I think the smell was quite agreeable.
Doing it outside will be fine assuming your not looking to produce Kg's of the stuff.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Ammonium nitranilate. Also sheds some light on the bonding/structure in nitralinic acid:
http://journals.iucr.org/q/issues/1964/03/00/a04104/a04104.p...
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
That journal requires subscription Taoiseach.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
There it is 
Unfortunately no synthesis is given, only a reference to Berichte der Deutsche Chemische Geselschaft. Most probably they made it from nitralinic acid,
not the bisodium salt.
Regarding the chloranile synthesis, is it possible to purify OTC paracetamol by dissolving in EtOH (12g should dissolve in 80ml EtOH) and precipate
with water?
[Edited on 9-11-2008 by Taoiseach]
Attachment: a04104.pdf (710kB)
This file has been downloaded 1070 times
|
|
|
SilencePlease...
Harmless

Posts: 5
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
In reply to my above paranoia, chloranil via paracetamol and NOCl proceeded well. Regrettably only about half the mass of paracetamol converted to
chloranil. The vessel, unlike Axt's became thick with ppt, and had slightly orangey lumps.
I shall try again.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Taoiseach
There it is 
Unfortunately no synthesis is given, only a reference to Berichte der Deutsche Chemische Geselschaft. Most probably they made it from nitralinic acid,
not the bisodium salt. |
Ammonium nitranilate is also described in Ann. 215, 140 by Nietzki as pretty barely soluble leaflets. This reference is also mentioned here, where it was obtained by neutralizing the acid with ammonia.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
I just made some chloranil from paracetamol+NH4NO3+HCl. It worked niceley, altough the product is contaminated with occasional brown-black lumps. I
will try to recrystallize it from acetone.
Here's an interesting paper, unfortunately its in German and I cannot DL it: Nitralinic acid from chloranil.
http://www3.interscience.wiley.com/journal/112372484/abstrac...
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Hydrazinium nitranilate & other high-density energetic nitranilates:
http://www3.interscience.wiley.com/journal/121569461/abstrac...
Again I cant DL this shit...
I recrystalized my chloranil from boiling acetone. It gave nice dark-orange crystals which are easier to handle than the yellow powder I originally
obtained.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Taoiseach
I just made some chloranil from paracetamol+NH4NO3+HCl. It worked niceley, altough the product is contaminated with occasional brown-black lumps. I
will try to recrystallize it from acetone.
Here's an interesting paper, unfortunately its in German and I cannot DL it: Nitralinic acid from chloranil.
http://www3.interscience.wiley.com/journal/112372484/abstrac... |
That's from the Ber. 20, 2027-2031. See page 1.
| Quote: | Originally posted by Taoiseach
Hydrazinium nitranilate & other high-density energetic nitranilates:
http://www3.interscience.wiley.com/journal/121569461/abstrac...
Again I cant DL this shit...
I recrystalized my chloranil from boiling acetone. It gave nice dark-orange crystals which are easier to handle than the yellow powder I originally
obtained. |
That's quite interesting; the hydrazine salt with a VOD very close to RDX. PCJ also very close 31.7 GPa (RDX: 33.8 GPa).
I was also reading in Liebig's Annalen (1892), p.15 about a compound, hexachloro-p-diketo-R-hexene made from chloranil, HCl + MnO2, or from
p-aminophenol by action of Cl2 (procedures in that ref). If this could have the Cl2 atoms replaced by -N3, then the compound
hexaazido-p-diketo-R-hexene would have the same C and O content as the title compound, but two more azido groups making it more energetic.
[Edited on 27-12-2008 by Formatik]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
The second article
referred to by Taoiseach is attached. Quite the mix of computational and synthetic chemistry, it was.
sparky (~_~)
Attachment: nitranilat.pdf (409kB)
This file has been downloaded 2078 times
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
The NH4-nitranilate synthesis worked but the yield was pathetic. Orange crystals that turn brown on drying. Deflagrates on ignition.
Anyone tried the silver salt yet?
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sparkgap
referred to by Taoiseach is attached. Quite the mix of computational and synthetic chemistry, it was.
sparky (~_~) |
Wow, thieves.
My preparation posted in 2007:
<i>"2g of chloranil was stirred into a solution of 5g of sodium nitrite in 200ml of water. Gradually all the chloranil went into solution giving
it a deep orange colour. The solution was left to boil to a volume of 100ml then cooled to 5°C whereby a yellow orange precipitate formed. This was
filtered and dried. Yield was ~1g."</i>
Their preparation published 2008, citing Z. Naturforsch. B: Chem. Sci. 1994, 49, 1021 – 1030.
<i>"Chloranil powder (2 g, 8.1 mmol) was added at room temperature to a solution of sodium nitrite (5 g, 72 mmol) in water (200 mL). The
reaction mixture was gradually heated to reflux. After stirring for 2 h at reflux, the clear deep orange reaction mixture was concentrated to 100 mL,
and then cooled to 5°C where a yellow-orange crystalline precipitate was formed. It was collected by filtration, washed with cold water (20 mL),
dried in vacuum to afford the product (1.2 g, 54%). No further purification was needed."</i>
Also mine:
<i>"I've previously attached its solubility data for the Na/K salts here, which is 0.724g/100ml @ 30°C and 0.567g/100ml @ 30°C
respectively."</i>
Theirs:
<i>"The solubilities of potassium and sodium nitranilates at 30°C are only 0.724 and 0.567 g per 100 g H2O, respectively."</i>
All literature preps I'd seen used a non-aqueous solvent for chloranil during the prep so unless theres some freak coincidence that the Z.
Naturforsch. B: Chem. Sci. article happened to have what I posted here near word for word .... Oh well, imitation is the sincerest form of flattery

[Edited on 30-12-2008 by Axt]
|
|
|
Plasmapyrobattics
Harmless

Posts: 10
Registered: 29-12-2008
Member Is Offline
Mood: Plasma...
|
|
Hi Axt
I’ve been here long time only as a reader and I enjoy your posts very much, since they are very professional, stimulating and enjoyable (shared
together with a few other great posters / people here). Today I decided to join in.
Indeed I agree here that someone might have been copying your work above as mentioned, but I suppose that happens. I also would feel the same about
imitation. Excellent indeed.
I hope not to be somewhat off-topic, but I would like to add / ask something about Trichloroisocyanuric acid (and its salt, Sodium
dichloroisocyanurate) – since we are discussing halogenated molecules like Chloranil and the effect of Sodium Nitrite on it (which leads to
interesting and practical results indeed).
Question : What is the prospects of adding Sodium Nitrite to an aqueous solution of Sodium dichloroisocyanurate (a relatively available Pool Shock
Treating Agent) ? Is there a reasonable chance to obtain Sodium dinitroisocyanurate ?
Or otherwise : Dissolving powdered Trichloroisocyanuric acid [Pool Chlorinating agent / Chlorine Pills] in water (containing HCl), and adding excess
Sodium Nitrite – to possibly (maybe) obtain Trinitroisocyanuric acid ? My guess is that both these suggested materials (results) might hydrolyze too
easily and thus decompose. Am I wrong ?
What is your views on this ?
Thanks
Plasmapyrobattics
|
|
|
| Pages:
1
2
3 |