| Pages:
1
2
3 |
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
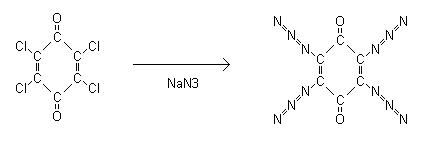
2,3,5,6-Tetraazidobenzoquinone
I come across this in a review article regarding organic azides and looked like a possible and more readily available alternative to cyanuric
triazide, there was only a passing mention of it which said it was investigated for its use as an explosive with reference to Sorm, F.: Chem. Obzor.
14, 37 (1939) or Chem. Abstracts 33, 7286 (1939), another reference on its preparation was given as <a
href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=9319&pid=109472"></a> its mentioned but I couldn't find
the prep in that article. 2,3,5,6-tetraazidobenzoquinone is formed by the reaction of sodium azide with 2,3,5,6-tetrachlorobenzoquinone aka chloranil.
I had a look for preparations of chloranil in the literature, and there is a lot, too many in fact so its hard to pin point the one most convenient to
the amateur. Urbanski volume 1, in the section on picric acid mentions:
"When reacted with chlorine, aqua regia or potassium chlorate in the presence of hydrochloric acid, picric acid yields chloranil along with
chloropicrin."
The picric acid route looks easy enough but simpler precursors may be had, this from MERK:
"Prepd (chloranil) from p- phenylenediamine or phenol by treating with KClO3 and HCl. Because of its great resistance to further oxidation, chloranil
is formed as the final product of the chlorate-HCl oxidation of many aromatic compds. For comprehensive list see Hunt ress, Organic Chlorine Compounds
(New York, 1948). Laboratory procedure starting with phenol or p- chlorophenol: Fierz-David, Blangey, Grundlegende Operationen der Farbenchemie
(Vienna, 5th ed., 1943) p 140."
The two examples are not readily available but what of the "many other aromatic compounds" Aniline likely would as well. Possibly p-dichlorobenzene?,
need a use for that crap. If not p-DCB then nitration to activate one of the chlorines then remove with NaOH giving mainly 4-chloro-2,6-dinitrophenol,
its known that picric acid and p-chlorophenol can be oxidised/chlorinated to chloranil so that probably has merit if p-DCB itself is unreactive.
I requested another article regarding the oxidation/chlorination of phenol and hydroquinone with HCl-H2O2 to chloranil, retrieved by solo <a
href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=9319&pid=109471">here</a>.
[Edited on 7-11-2007 by Axt]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Axt
Because of its great resistance to further oxidation, chloranil is formed as the final product of the chlorate-HCl oxidation of many aromatic compds.
For comprehensive list see
Huntress, Organic Chlorine Compounds (New York, 1948). |
Wonder if some common materials like aspirin , salicylic acid ,
and methyl salicylate could be on that list .
It might be interesting to substitute sodium tetrazylazide
(sodium 5-azidotetrazole) for the sodium azide 
Alkaline hydrolysis of tetracene using NaOH should provide the sodium 5-azidotetrazole , with expulsion of ammonia
as a byproduct . See the first page of the sticky thread on
5-ATZ related materials , about halfway down the first page .
http://www.sciencemadness.org/talk/viewthread.php?tid=8144
That should yield a tetra-tetrazylazide ( C10-N28-O2 )
( bet you can't say its name without stuttering ) 
I'll leave that tongue twister to somebody else 
[Edited on 7-11-2007 by Rosco Bodine]
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
Here are some US patent numbers that seem among the best for the home production of chloronil:
US5334735
US2872461
US2519319
US2414008
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It looks to me like the H2O2 + HCl reaction might be applicable to the common and cheap analgesic paracetamol , ( "tylenol" , acetaminophen ,
p-acetamidophenol ) to produce chloranil .
I haven't yet found a reference directly for this suspected
reaction , but it seems the most likely approach using OTC
precursors .
[Edited on 7-11-2007 by Rosco Bodine]
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
The main disadvantage of C6(N3)4O2 is its decay at RT (mass loss 4.9%/10days). Interesting, can it be converted, for example, -> C6(N3)4(=NOH)2 or
C6(N3)4(=NNH2)2 in order to increase the stability?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I wonder .....does this exist , is it already known ?
It sure seems likely , even straightforward .
2,3,5,6-tetra-tetrazylazidobenzoquinone , or
2,3,5,6-tetra-tetrazolylazidobenzoquinone
Wonder what its properties would be ?
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
I wonder .....does this exist , is it already known ?...
|
If you mean C6(N3)4O2:
Blue non-volatile cryst., 10mg det. at 91C (5K/min), sol.in acetone, spar.sol.EtOH, insol.H2O. High friction sensitivity, impact 0.5kg*0.2m, expl.in
flame. 20mg, pressed at 50-100MPa, initiated TNT.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The two examples are not readily available but what of the "many other aromatic compounds" Aniline likely would as well. Possibly p-dichlorobenzene?,
need a use for that crap. If not p-DCB then nitration to activate one of the chlorines then remove with NaOH giving mainly 4-chloro-2,6-dinitrophenol,
its known that picric acid and p-chlorophenol can be oxidised/chlorinated to chloranil so that probably has merit if p-DCB itself is unreactive.
|
Just mono-nitrate it and then reduce to 2,5-dichloro-aniline which should oxidise to the quinone.
It may be possible to combine the HCl-H2O2-MgCl2 reaction with a Fenton-style oxidation of p-DCB by adding a trace of Fe(II) or copper salts. The
Fenton puts a HO- onto the aromatic ring, that phenol then gets taken to chloranil. Too much Fenton activity will result in complete oxidation of the
aromatic substrate, so it would be a balancing between the two reaction paths.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
We need some Belgian input here 
Where's Philou Zrealone when you need him ?
Need a nice clean dioxin free chloranil synthesis ,
from OTC materials so how about it ?
Still can't find any reference to my above named
tongue twister . Any predictions on its density
and stability , sassiness ?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
On the tongue twister, I can't see it being too high density with all the long tetrazyl azide groups hanging off with all sorts of deviations from
planarity. How accurate is chemsketch's density predicter anyway?(this molecule is too big for it).
I cannot see it not being extremely frisky...following the general increase in friskyness from azide to tetrazylazide.
EDIT for clarity: I think it would be more powerfull than the simple azide flavour, but I worry about how stable to minute stimulus it would be.
Now hanging some nitrotetrazoles off there...
[Edited on 10-11-2007 by The_Davster]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Hmmmm ...I was thinking high density octagonal plates
might be possible with adjacent molecules interlocking like pairs of 4 projection castle nuts . And yeah there are other interesting possibilities .
I'm not sure about the
nitro variant ...IIRC the nitro group can actually be a diluent in tetrazole energetics , in contrast with its
more usual effect .
I don't follow what you are saying about the power ,
as it would for damn sure be stronger than the azide ,
just going by the nature of the additional nitrogen where tetrazole and azide are combined could give a near hundred percent increase over azide alone
. Tetrazylazides are described as "extremely brisant" .
I was thinking this could be a non-metallic primary .
It might also plasticize with ethyleneditetrazylazide .
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The tetraazide has an entry in PATR2700 under benzoquinone and derivatives.
The stability info artem provided kinda rules out practical usage, even though its initiating ability is very high. Where did that info come from
artem?
The instability isn't mentioned in PATR2700 although it does say that it dissolves with decomposition in organic solvents which would make conversion
of the carbonyl oxygens to the hydrazone/oxime difficult. Also the chlorines in 2 and 5 positions are very labile, as is the azido groups, thus
reaction with the amines would likely replace those in preference to, or as well as the oxygen.
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
..Where did that info come from artem?.. |
This is from hand-book (Chmelnitsky); the origin - previously mentioned F.Sorm. Chem Obzor.
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Found a second entry in PATR2700 which does mention its instability, pg T71 "decomps in air forming a yel-grn powder which when touched with a flame,
ignites but does not deton as does the original compd".
But anyway, heres the french citation that gives the synthesis of mono,di and tetrazidobenzoquinones. Havn't tried to read it but I ocr'ed it for
pasting into google translator.
Attachment: azidobenzoquinones - BullFr, 35,1190 (1924).pdf (753kB)
This file has been downloaded 1451 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The attached article mentions the effect of aqua regia on many organic substances with chloropicrin and chloranil being the major products.
Aniline only gives a small yield with much tarry prducts and no other convenient precrsors were described but some were hinted at, namely aspirin and
paracetamol which rosco mentioned. The most simular compound to paracetamol, p-aminophenol is mentioned to give a good yield withoud tar. Heres some
select extracts;
"It (chloranil) has been obtained by the action of a mixture of hydrochloric acid and potassium chlorate on aniline, phenol, salicylic acid,
nitrosalicylic acid and dinitrosalicylic acid."
"By the action of aqua regia, which has a far greater oxidizing and chlorinating action than potassium chlorate and hydrochloric acid, on aromatic
compounds, chloranil is very frequently formed ; and as chloranil itself breaks up into chloropicrin by the action of aqua regia, this is also a
general product of such reactions."
"In the case of hydroxy and amino substituted products, chloranil is formed, but is accompanied by a large amount of tarry and other secondary
products."
"p-Aminophenol- On adding p-aminophenol to a mixture of nitric and hydrochloric acids, at first there is no action, but soon afterwards the action
commences with frothing. The mixture is then warmed on the water bath for two hours, and at the end of the operation a sulfur yellow crystalline
product is produced. In this case no tarry product is formed. The yellow product obtained is crude chloranil which is purified by sublimation and is
found to melt at 289° in a sealed capillary tube."
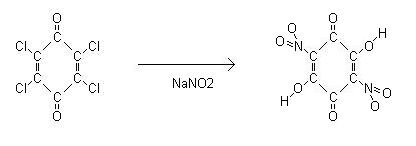
Chloranil can also be used to prepare nitranilic acid.
Chloranil according to ber, 20, 2027-2031 (1887) when treated with an aqueous solution of NaNO2 gives nitranilic acid, (C(OH)-C(O)-C(NO2)=)2, this is
mentioned in PATR2700 D1290 though through a different route. Its lead salt "explodes on impact or frictional influences" referencing to german patent
DE407416.
[Edited on 3-12-2007 by Axt]
Attachment: chloropicrin and tetrachlorobenzoquinone from aqua regia- JACS,1916; 38(9); 1813-1821.pdf (625kB)
This file has been downloaded 1736 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
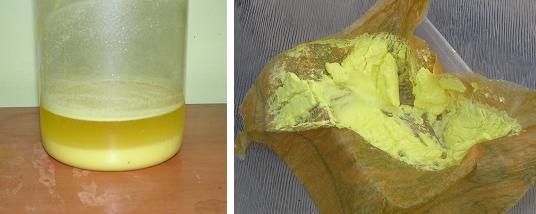
The action of aqua regia on paracetamol closely met the description of that of p-aminophenol as posted above. On mixing 15g paracetamol with 100ml 32%
HCl, then adding 50ml 70% HNO3 after a short time a rapid reaction commenced with much foaming. After this died down the mixture was heated @ 80°C
for 2 hours whereby the evolution of gasses had ceased. A sulphur yellow precipitate remained.
[Edited on 4-12-2007 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
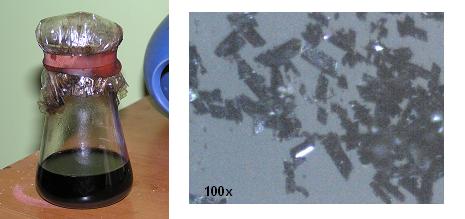
1.6g of the supposed chloranil obtained from paracetamol was suspended in 28ml of ethanol (as methylated spirits). Into this was added 1.9g of sodium
azide and the solution swirled for two hours. The mixture quickly turned orange then reddish brown then finally black. It was left sit overnight
(~8hr) then poured into 200ml water, swirled and filtered. Obtained was a bluish-black crystalline substance.
Small quantities amounting to about 1/4 matchhead were scooped onto the back of separate matches for drying, and the remainder on the filter paper was
flushed into the lawn with water to dispose of it.
Even when wet, the 1/4 matchhead of tetraazidobenzoqunone would detonate with a sharp ear ringing report when touched with a flame.
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Sodium nitranilate synthesis was reported in <a
href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=9319&pid=111612">this translation</a> of ber, 20, 2027
(1887). They added a saturated solution of chloranil in acetone to a concentrated solution of NaNO2 in water. The problem is a saturated solution of
chloranil is about 4g in 150ml acetone thus on addition to the smaller volume of NaNO2/water presumably you would get a precipitate of NaCl. I tried
to simplify this by just boiling chloranil (which was already a very fine powder) in aqueous NaNO2 until the sodium nitranilate went into solution ,
then cool to precipitate it. I've previously attached its solubility data for the Na/K salts <a
href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=433&pid=69514">here</a>, which is 0.724g/100ml @ 30°C
and 0.567g/100ml @ 30°C respectively.
2g of chloranil was stirred into a solution of 5g of sodium nitrite in 200ml of water. Gradually all the chloranil went into solution giving it a deep
orange colour. The solution was left to boil to a volume of 100ml then cooled to 5°C whereby a yellow orange precipitate formed. This was filtered
and dried. Yield was ~1g.
0.4g of the presumed sodium nitranilate was dissolved into 100ml of boiling water. Into this was added 0.6g lead acetate in 20ml water. A dark reddish
orange precipitate formed which was filtered and dried.
The lead nitranilate took the form of small copper coloured needles which in pinch sized quantities detonated with a loud report when touched by a
flame.
The pictures below are sodium nitranilate of filter paper, the precipitate of Pb nitranilate and the lead salt under microscope. The bottom row is the
ignition of Pb nitranilate.

|
|
|
arkansas
Hazard to Others
  
Posts: 133
Registered: 13-12-2006
Member Is Offline
Mood: No Mood
|
|
On the explosive properties of tetraazido-p-benzoquinones
William H. Gilligan and Mortimer J. Kamlet
Tetrahedron Letters, Volume 19, Issue 19, 1978, Pages 1675-1676
The diazido-dichloro-p-benzoquinone (II), while it does not explode upon heating, does have an impact sensitivity of 14 cm. This indicates that II is
a sensitive explosive despite Winkelmann's contention that it has no explosive properties. It should be handled with due caution.
Attachment: tl1978.pdf (102kB)
This file has been downloaded 1353 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
Sodium nitranilate synthesis was reported in 1887
|
1887 ! Wow
I new I'd seen this before, here's a more recent preparation from
CIA Field Expedient Methods of Explosives Preparation
Chloranil :
A slurry of 5.0 grams of salicylic acid and 100 ml of concentrated hydrochloric acid was
heated to 80ºC and 5.0 grams of potassium chlorate added in portions ( with effervescence ).
An additional 400 ml of concentrated hydrochloric acid and 5.0 grams potassium chlorate was
added and the mixture allowed to heat at 80-90ºC for four hours. After filtering, washing with
water and air drying, the yellow crystals melted at 190-200ºC ( sealed tube ). The yeild was
5.45 grams.
Sodium Nitranilate :
A mixture of 5.0 grams of chloranil and 200 ml of ethyl alchohol were heated to boiling and
treated with a solution of 5.6 grams of sodium nitrite in 100 ml of ethyl alcohol. The mixture
was heated with stirring for one hour and allowed to cool. The orange-gold crystalline product
was collected on a filter, washed with ethyl alchohol and air dried. Yeild was 1.85 grams.
Lead Nitranilate :
A solution of 1.0 gram of sodium nitranilate in 100 ml of boiling water was filtered and the
filtrate treated with 2.9 grams of lead nitrate in 10 ml of water. The mixture was stirred for
one half hour and the gold platelets collected on a filter and washed with water. After drying
at 80ºC for three hours, the product weighed 1.23 grams. The product ignited with a loud
report on flame contact.
__________________________________________________________________________
Yes I know, not the most reliable source but it approximately corresponds to your experience.
Someone went to a lot of trouble working this up and it's not a commonly seen recipe.
The Desert publication is from 1977 and the text dates to well before that.
Back when this was contrived using Salicylate, Acetaminophen was not OTC but by presciption only ,
called Dilone for mitigating menstrual cramps. Interestingly investigation of it also dates to the late
19th century.
| Quote: | Originally posted by Rosco Bodine
It looks to me like the H2O2 + HCl reaction might be applicable to the common and cheap analgesic
paracetamol to produce chloranil .
|
| Quote: | Originally posted by Axt
The action of aqua regia on paracetamol closely met the description - as posted above
|
.
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Indeed that method in FEMEP has its basis in journal literature, but I have some issues with it.
| Quote: | | Back when this was contrived using Salicylate, Acetaminophen was not OTC but by presciption only |
Actually over 100 years earlier, the first report of the oxidation/chlorination of salycilic acid to chloranil using KClO3-HCl was in <i>Annalen
der Chemie</i>, 78, 4 (1851). Which is well before even aspirin was patented. Though the melting point given for chloranil in FEMEP is 100°C
too low!
Also, while ber, 20, 2027 (1887) also mentions ethanol as being a suitable solvent it pours this into an aquous solution of NaNO2. I'm not totally
sure of the mechanism of this reaction but the only way I can see the hydroxy groups being added is via the reaction of the chlorine or nitro
substitute (ortho to the other nitro groups) with water. With only ethanol I'd expect a phenetol derivative.
With mine, after filtering the tiny copper coloured needles and leaving the yellow solution sit, a further small crop of crystals did form in the
solution which met the description in FEMEP, golden plates. Though on filtering and drying these they would only pop and splutter when exposed to
flame. There is only a description of the basic lead salt in PATR2700 which is said to be red. Presumably I had the normal salt.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Great stuff!
I don't understand why you insist on these OH's being present at two positions, where did you hear of this?
Why would the tetraazido form exist, but not the tetranitro?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That possibly novel compound I suggested above ,
2,3,5,6-tetra-tetrazolylazidobenzoquinone
represents something of a curious physiological anomaly ,
given that it is both a tongue twister .......
and a fanny puckerer simultaneously   
Sorry , I know that's bad .....
but I just couldn't resist 
Anybody run a simulation model on that one ,
any predicted density , energy , ect . ??
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
I don't understand why you insist on these OH's being present at two positions, where did you hear of this? |
Oh its not me that insists on 2 OH's! thats simply the known structure of nitranilic acid, and I only (well, nearly) followed the known synthesis for
this. If "tetranitrobenzoquinone" were aromatic this would be the expected reaction where nitro groups ortho to each other being unstable, being
replaced by hydroxyl group via water. See <a
href="http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=DE407416&F=0&QPN=DE407416">DE407416</a>, I dont know if theres anything
interesting in there but you can read it and its the only known reference to the lead salt of nitranilic acid.
Also probable are peroxidic explosives derived from chloranil. There is reference to a tricyclic C6O6 (imagine the most obvious product of Na2O2 with
chloranil) in the following article. Though I suspect it is just a theroretical study.
Gernot Frenking "The Structure of Cyclic C6S6 and C6O6" <i>Angewandte Chemie International Edition</i>
Volume 29, Issue 12, 1990, Pages: 1410-1412
And
Fariba Nazari "Stable structures of oxocarbons and pseudooxocarbons of group VI" <i>Journal of Molecular Structure: THEOCHEM</i> Volume
760, Issues 1-3, 2006, Pages 29-37
Surprise surprise, your compound is not listed in scifinder Rosco, I'm shocked  Actually there was no tetrazole derivatives listed.
Actually there was no tetrazole derivatives listed.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Axt
Surprise surprise, your compound is not listed in scifinder Rosco, I'm shocked  Actually there was no tetrazole derivatives listed.
Actually there was no tetrazole derivatives listed. |
Maybe it's an unlisted experimental roo medicine 
|
|
|
| Pages:
1
2
3 |