| Pages:
1
2 |
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
And that gives me rough estimates of 22,000 psi on the low end and 28,000 psi on the high end. Nice!
For the autoclave at 180 C and 40% fill, I got somewhere halfway between 0.1 MPa and 4 MPa - it's a little harder to interpret this gap
logarithmically, but I'd guess it's pretty close to 1 MPa like you said.
Still working out a good price for the pressure vessel I want to build - might have to scale it back a bit to keep costs under the thousands,
unfortunately. And then there's the matter of sealing it...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | And that gives me rough estimates of 22,000 psi on the low end and 28,000 psi on the high end. Nice!
For the autoclave at 180 C and 40% fill, I got somewhere halfway between 0.1 MPa and 4 MPa - it's a little harder to interpret this gap
logarithmically, but I'd guess it's pretty close to 1 MPa like you said.
Still working out a good price for the pressure vessel I want to build - might have to scale it back a bit to keep costs under the thousands,
unfortunately. And then there's the matter of sealing it... |
I don't know if you can use the graph that way. I think the vertical line should be interpreted as one of two phases the bottom being gas and the top
being liquid and undefined in between.
My intention was that you only use the graph for the super critical phase when both the pressure and the temperature is above critical point. You
should use the steam tables for that combination of temperature and pressure if only because its more accurate.
The reason you can use the fill factor at RT for the supercritical phase is because that tells you the density of the super critical .phase if it
occurs but that is not necessarily true below the critical point when two phases are present in equilibrium.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I discovered one of my SodaStream bottles has the following stamped on it
Sl 83 BS 5045 CO2 TP250 BAR
Its tempting to think that does mean a test pressure of 250Bar. Thats high for CO2 considering fire suppression CO2 bottle are tested to 30 bar.
Perhaps SodaStream really do not want them exploding.
BS5045 is a specification for gas bottles. It forbids any welding on the bottle. Probably because the bottles are tempered steel. So no cutting the
end off one and welding a flange on. So to seal the end of a bottle that has one end cut off it will have to be clamped between two plated with a
suitable number of scheduled bolts holding the plates together. The end of the bottle could be lapped to its plate perhaps with the plate having a
groove to fit the bottle and a copper gasket. The scheduled bolts could be used and perhaps tightened to a certain torque such that the seal is
broken at max pressure. A built in pressure relief valve which combined with pressure water testing should make it reasonable safe. A hole in the
ground test chamber should still be used.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Quote: Originally posted by elementcollector1  | If I recall correctly, they never mentioned the fill rate for any of their experiments, so you're quite likely to be correct. Is there a way to
calculate how much pressure is generated, given, say an 80% fill of the tube at the start?
@Doped-Al2O3-fusion: Have you considered getting a specimen of iron pyrite for comparison under the microscope? |
Yes I have. I do have some pyrite/marcasite which I gathered from the river around where I live. I have one specimen with loads of it peppered all
throughout the rock and it's probably the prettiest sample I've personally found so far. I still want to buy some from eBay because everything I have
is very small and mostly visually bland. Most of what I have is the size of sand grains. I'll get a picture up of some of what I have when I get a
chance.
I still have four five gallon buckets full of rocks and sand that I've not had a chance to sort through yet. I was supposed to go up to the Skycomish
river today and collect rocks and sand, but my back is not cooperating with me at all. It's supposed to have decent specimens of pyrite so I might try
to just fight through the pain as long as I can, or just wait until tomorrow. I really wanted to go this weekend so I'm keeping my fingers crossed.
I'm currently running my autoclave with some of that sodium thiosulfate pentahydrate I purchased along with some freshly prepared ferrous sulfate
heptahydrate. I used a layer of diatomaceous earth separating the thiosulfate layer on bottom and ferrous sulfate on top. I figure the diatomaceous
earth should be inert enough for this use. It is basically a reaction timing buffer and wicking agent for the thiosulfate. Once the vessel is hot
enough the thiosulfate will liquify in its water of crystallization. This liberation of H2O allows the sodium thiosulfate to wick into the
diatomaceous earth and make contact with the Fe(II) ions from the ferrous sulfate and begin the hydrothermal reaction.
Last time I used an aqueous solution of ferrous sulfate, sodium thiosulfate and sodium polysulfide(s). It instantly forms iron sulfide which
precipitated out as a black sludge. It honestly doesn't look much different than the end product containing the iron disulfide. It can be sorted out
with HCl because FeS2 won't react with it. The FeS and other byproducts do dissolve in HCl but will also precipitate elemental sulfur and produce H2S.
Once fully reacted, filter out the black sludge which should be FeS2 & sulfur. Heat the black sludge in xylene or toluene to dissolve the sulfur
and decant as much solution as possible. Repeat several times then hot filter through a Buchner funnel if you have the option of glass fiber filters.
I don't imagine trying to unclog sulfur out of a fixed course glass filter type funnel would be all that fun.
The end product should not turn brown within the immediate future if it is FeS2, nor will it be ferromagnetic. If a layer is placed on a piece of tape
it's electrical resistance can be measured and compared to that of natural pyrite. FeS2 should also be contained inside Energizer lithium batteries
and that could be a source to compare resistance test results.
Unfortunately, using the microscope can't confirm if the product is FeS or FeS2. I'm hoping to find a way of producing visually observable cubic
crystal structures. I highly doubt I can make that happen based off all the research I've done, however I've only just begun my journey of failing.
After enough fails, I might find success.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I was going through my first batch of synthesized FeS2 which I hadn't found any indication of any visible cubic crystal structures...until just now. I
have mixed feelings on this as I am heavily scrutinizing what I am observing, and I sort of begin to doubt myself as some accidental contamination.
Anyhow I will be analysing my 2nd batch shortly. I just wanted some side by side comparison pics. The new batch may be foo difficult to distinguish
any iron sulfide formation due to using the diatomaceous earth as an inert media inside the reaction vessel.
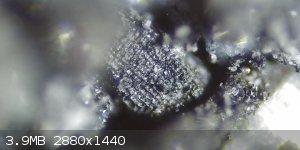
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here's an update on the FeS2 synthesis. The picture of the two black piles is of the 1st and 2nd batch of FeS2 I synthesized. The first batch is on
the right side. The second batch may have some successful results, but I've not had a chance yet to test it out yet. I can't tell much about it
under the microscope since I used diatomaceous earth and an intermediate substrate to buffer the timing of the reaction once the vessel was up to
temperature and pressure. So, due to that deliberate contamination, image analysis under the microscope is a bit pointless as far as I'm concerned.
With the aforementioned said, I have a 3rd batch that I made with no diatomaceous earth. I also added elemental sulfur and a small amount of NaOH.
The autoclave is still in the oven at home and I'll open it up tonight after work.
I've also included some pictures of natural pyrite I've collected from the Snoqualmie river. The rock with the single visible crystal in the middle
was one I found just yesterday. I've got another angle to show another crystal on the top side and in that picture you can see a different angle of
the single crystal. The other rock with pieces of pyrite scattered throughout was one I found last year by chance. I didn't even know there was
pyrite in that rock until I crumbled part of it off after having bathed the specimen in HCl for a few days.
   
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here are some pictures from my last batch (batch #4). This is probably the reason you don't see other people posting any pictures at all of synthetic
pyrite.--unless you have an electron microscope.
These micrographs are post treatment with hydrochloric acid and boiled in toluene. FeS2 is completely insoluble in both
I'm not sure what I'm going to end up doing with this stuff in the end.
    
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here are my batch #5 results. I'm debating uploading a video to my YouTube channel upon first investigation of the contents. I thought it was a
failure from my first observations, but as I emptied the contents of the reaction vessel, I was finding some interesting detail under the microscope.
I still need to perform the cleaning steps which will eliminate all other products while leaving the dark sludge of hypothetical FeS2.
I need to perform some additional research because I want to be sure I've eliminated the possibility of some other unaccounted for end products from
this hydrothermal reaction. I know definitely it isn't iron sulfide (FeS) as that dissolves readily in HCl. Strongly heating the remaining sludge in
toluene does a fantastic job in removal of any excess sulfur. I'm very well aware that my product may not be of the pyrite structure, but rather
marcasite.--chemical composition is the same. I also ruled out pyrrhotite because the product I have shows no signs of magnetic properties.
*edit*
I'm well aware that the pictures aren't the greatest, but I didn't have time the other night to setup my computer with the microscope mounted
camera.--Windows updates screwing life up as usual.
      
[Edited on 2-10-2018 by Doped-Al2O3-fusion]
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Isn't FeS soluble in HCl?
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Yes, exactly. Any FeS which might be present in solution will be dissolved by the HCl and emit H2S as a byproduct which I can test using lead
acetate. Once my product is thoroughly washed with HCl, I take an additional small sample and place a drop of 32% HCl onto it and if that doesn't
emit any H2S, then there is no FeS present in the product. FeS2 won't dissolve in HCl.
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Doped-Al2O3-fusion  |
Yes, exactly. Any FeS which might be present in solution will be dissolved by the HCl and emit H2S as a byproduct which I can test using lead
acetate. Once my product is thoroughly washed with HCl, I take an additional small sample and place a drop of 32% HCl onto it and if that doesn't
emit any H2S, then there is no FeS present in the product. FeS2 won't dissolve in HCl. |
Ah sorry I
misread/misunderstood your sentence, my bad
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
[rquote]Ah sorry I misread/misunderstood your sentence, my bad [/rquote] [/rquote]
No worries. I do that all the time too.
[Edited on 2-10-2018 by Doped-Al2O3-fusion]
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I've refined my Batch05 of FeS2 and found some very interesting and exciting results. I found actual cubic crystals, so this would indicate the
pyrite structure I was aiming for was created along with quite a bit of marcasite. These structures were found in bubble/nodules which had formed on
the inner wall of the reaction vessel and these can be observed still in the vessel in previous photos from my last picture update. It's like tiny
geodes full of FeS2 crystals.
This batch underwent 24 hours in a 32% HCl bath on magnetic stiring. It was thoroughly rinsed eight times with distilled water. It was then
processed through several acetone washes and completely dried at 120 degrees Celsius (approximately 30 minutes). Once it was dried, the solid was
placed in a 100mL Erlenmeyer flask with xylene and heated to a near boil for one hour, then hot vacuum filtered. This step was repeated a second time
to ensure any trace of elemental sulfur was removed. Again the solid was dried after the xylene wash, rinsed three times again with acetone, followed
by three heavy rinses of distilled water.
Quite a bit of my product is still stuck in the 1.6 micron glass fiber filter paper (three filters) and I can't figure out how to get it thoroughly
removed from the glass fibers. Maybe there is a better brand than the Omicron filters I'm using, or maybe there is a better material fit for the job
so that more of the solid can be successfully collected. Please let me know of any suggestions for different 47mm filters.
To any extent, I'm fairly certain batch05 was a great success. The particles show no response to magnetic fields, so that rules out most, if not all
of the other possible byproducts, especially after the HCl bath and organic solvent washes.
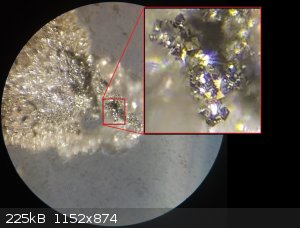    
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Further experimental update.
This most recent experiment appears to be a failure in an attempt at forming FeS2. Tetrasodium EDTA was used in solution during this run to see what
effect it would have on crystal formation with previously successful solution recipes. I felt using a chelating agent might slow the rate of crystal
formation and result in more even crystal structure. My notes post-reaction are below.
0.4g anhydrous ferrous sulfate in 10mL distilled water
0.2g elemental sulfur (finely powdered)
1.0g Na2S2O3 (sodium thiosulfate)
0.025g EDTA (tetrasodium ethylenediaminetetraacetic acid)
0.2g NaOH
-Seeded solution with small amount of FeS2 formed from last experiment.
-Reacted in autoclave at 170 C for 12 hours and allowed to cool in oven until room temp.
-Checked pH upon opening vessel and noted that it dropped from the initial 13 starting pH to approx 11.5.
-Noticed small amount of reddish brown particulates floating on surface of solution, which is safe to assume to be Fe2O3.
-Poured solution into glass beaker and noted greenish black color of solution and not much in the way of any visible solids.
-Sitting in beaker for 20 minutes and the solution is still very uniform, so formed solids are colloidal. Also noting that the solution is not
turning red/brown on the surface as it sits in beaker with outside air blowing from the window. My experience with ferrous hydroxide is that it
oxidizes almost immediately on the surface forming a reddish brown coloration upon exposure to atmospheric oxygen.
-My guess, due to the dark green color of solution, most of the ferrous sulfate was converted to ferrous hydroxide with very little interaction with
the sodium thiosulfate.
-There may be some ferrous sulfide as the sodium thiosulfate, elemental sulfur and sodium hydroxide most likely formed some amount of FeS.
-Sodium thiosulfate will prevent Fe(II) ions oxidizing to Fe(III), which is why I'm assuming that this is the reason for no noticeable surface
oxidation. It is at least delayed after nearly 30 minutes at this point.
-I'm guessing the NaOH and elemental sulfur combined to form NaS
-If the sodium thiosulfate was not decomposed and/or reacted with the Fe2SO3 to form FeS2, then I suspect the EDTA prevented much if any FeS2
formation.
********************************************************************************************
I will examine and test the solution to see if any FeS2 was formed and will update my results once available. Based on my previous five reactions,
this solution is visibly dissimilar from results of all previous products. Observing the pH to still be quite high is also a major clue that very
little reaction took place.
I'm also making my guesses on the reaction product(s) a bit early. It may turn out that I have very fine nano particles of FeS2, which will be
beneficial on planned future experiments, however, I was hoping with the seeded solution, that I may end up with larger cubic faced structures. Maybe
there is and I'm just being too impatient.
I'm more likely to die doing what I love. I'm a hobby chemist. I void warranties.
|
|
|
Texium
|
Thread Moved
29-11-2023 at 11:41 |
| Pages:
1
2 |