| Pages:
1
2 |
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Hydrothermal Synthesis of Sulfides
I'm in a side quest to create synthetic pyrite because I want to. Practicality is of no concern as I want to explore the science.
I'm able to produce crude synthetic ruby and sapphire through flame fusion. I'm working on a Verneuil furnace but this project is temporarally
stagnant at the moment due to finances and design considerations for producing 7 Liters per minute of gaseous fuel (hydrogen & oxygen) for the
necessary flame.
So, while I wait on improving the fuel production apparatus, I am working on side projects, which includes hydrothermal synthesis. I'd love to create
iron pyrite just to do it. I'm not expecting large cubic golden colored crystals, but something which can be verified even at the microscopic level.
I've got the microscope, chemicals and hydrothermal reaction vessel.
Only one thing holds me back on moving forward and that is fear of metal shrapnel flying out of my oven at great speed. I've got a basic idea on a
few formulations that I've been working on which will either use iron powder, iron sulfide, ferrous sulfate or some other ferrous salt I've not yet
considered. I plan to use hydrated sodium thiosulfate since this will liquefy in its own water of crystallization and provide a means to obtaining a
polysulfide. I figured in the addition of extra elemental sulfur powder and sodium hydroxide to prevent any acidification of solution during the
reaction as to restrict the production/evolution of S, SO2, H2S or other products of decay which can produce excessive gas pressure in the reaction
vessel.
If anyone has any experience with this type of synthesis, please feel free to lend any advice. I'm not scared of failure, but safety is a primary
concern.
I also didn't name the subject of this post strictly towards pyrite as there are many forms of pyrite. There are also other transition metal sulfides
which produce larger than nano-scale crystal formation without having to use extreme pressures, such as copper sulfide(s). I'm not sure which sulfide
I have created personally (covellite or chalcocite), but I have documented proof of generating faceted crystals with the reaction of sulfur and copper
(II) oxide in a test tube.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
nice project, i'm also intrested in hydrothermal crystal growing, and like you i'm a bit scared of a pressure vessel exploding so i haven't
experimented on anything yet.
i'd like to grow a cinnabar single crystal, but a bit bigger than microscopic 
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Quote: Originally posted by Ubya  | nice project, i'm also intrested in hydrothermal crystal growing, and like you i'm a bit scared of a pressure vessel exploding so i haven't
experimented on anything yet.
i'd like to grow a cinnabar single crystal, but a bit bigger than microscopic 
|
Your project sounds awesome! You probably have a better chance at growing larger crystals with HgS than I do with FeS2.
I have been working over some shredded steel wool in my ball mill and just filtered out the iron (steel) powder.

|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
What's the max pressure for your hydrothermal vessel? Most syntheses I've seen for quartz and others require an enormous pressure of 150 MPa to
achieve fast, good quality results. This means that most alloys will rupture unless the vessel is ridiculously thick.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Quote: Originally posted by elementcollector1  | | What's the max pressure for your hydrothermal vessel? Most syntheses I've seen for quartz and others require an enormous pressure of 150 MPa to
achieve fast, good quality results. This means that most alloys will rupture unless the vessel is ridiculously thick. |
I'll check the specifications*edit* specs below. I know I have them downloaded in .txt either on this laptop or my other PC. I'm definitely not
expecting anything spectacular or visible with the naked eye. I just want to see if anything microscopic is possible. Once I'm done exploring
formula options, I'll be conducting a test over a several day heating period.
The best I'm hoping for are microscopic crystals. I know that I'm not able to find any crystal structure whatsoever in plain FeS, so I don't have
high hopes with finding anything visible under the microscope for FeS2. However, the synthesis methods are quite different since FeS was done in both
a test tube and a larger batch in a stainless steel vessel burning in open air basically.
I was able to get visible crystal structures, at the microscopic level, for CuS from synthesizing in a test tube with continued heating for ten
additional minutes keeping it red hot. I used an aluminum foil plug to keep most air out during this synthesis as I also did with FeS.
If I get no visibly observed crystals from the hydrothermal synthesis, I'll measure electrical resistance & conductivity of the post reaction
compound and compare to several other samples used for control.
Specs:
Here are the basic reported specs by the eBay seller. I got the PPL liner and the 50ml version.
3. Main indicators
(1). Operating temperature: 260c
(2). Working pressure: 3MPa (gauge)
(3). Heating, cooling rate: 5 c / min
(4) specification 25,50,100,120,200 ml other customized according to user needs.
4 methods of operation
[Edited on 24-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here is a paper I found on the hydrothermal synthesis of transition metal sulfides. I thought up the use of sodium thiosulfate prior to finding this
paper, so that made me a bit excited that my mind was possibly in the right place.
http://www.ijnnonline.net/article_3959_3e68e38f9774ce71a192b...
I then also found this document:
http://www.ukm.my/jsm/pdf_files/SM-PDF-39-2-2010/13%20Fei%20...
[Edited on 24-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here is the actual vessel I purchased:
https://www.ebay.com/itm/50ml-PPL-lined-Hydrothermal-synthes...
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
@Doped-Al2O3-fusion: I've just read about the Verneuil furnace. It's really fascinating.
If you're worried about flying metal, perhaps a lexan sheet may help.
Check out this video. He fires rounds at it.
https://www.youtube.com/watch?v=NDSG0I8TFdk
Best of luck with everything. Grow some really cool stuff and show us. 
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I used 3.5g of iron powder and 2g of sulfur to make this batch of iron sulfide. Product yield was 5.243g. I should have probably used a slight
excess of sulfur to account for the sulfur dioxide formed during the reaction.

|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Quote: Originally posted by MJ101  | @Doped-Al2O3-fusion: I've just read about the Verneuil furnace. It's really fascinating.
If you're worried about flying metal, perhaps a lexan sheet may help.
Check out this video. He fires rounds at it.
https://www.youtube.com/watch?v=NDSG0I8TFdk
Best of luck with everything. Grow some really cool stuff and show us. 
|
Thank you. I just want to find something microscopic, but definitively a crystal lattice at best. These other researchers created nanoscale
structures in a matter of 90 minutes at approximately 180 C if I recall correctly. If I dedicate a minimum of 48 hours in the range of 200 C and 220
C, then I have hopes of generating larger structures. To create something visible by eye, I would be looking at 8 to 12 weeks at best, and even then
I would expect assistance with at least a magnifying glass.
As far as the Verneuil furnace, I can't wait to get back to work on that. I've got all these prepared containers of feed stock with variable amounts
of Cr, Fe and Ti doped aluminum oxide. My fixation on creating flame fusion rubies and sapphire was the inspiration for my username which I still
don't like. My own username sounds stupid to me. LOL.
Here is also a link to a shared google+ album I created a while back. It shows the crude ruby and sapphire I generated with a MAPP/Oxy torch. I
expected much carbon contamination and opaque crystals. I was able to keep the carbon contamination to a minimum surprisingly, which may have been
due to the number of heat treatments I applied to force the carbon out of the crystal as CO2 excrement.
https://photos.google.com/share/AF1QipPHzz6J_BrQmWVoBHtTp7hu...
[Edited on 24-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I'll most likely be using FeSO4 (heptahydrate) & Na2S2O3 (pentahydrate) in my reaction vessel. It seems this is the most accessible and sensible
method for the home chemist due to the lower temperature ranges from 130 C to 180 C. H2S would need to be generated to dissolve the FeS.
I'm glad I thought of using sodium thiosulfate and ferrous sulfate as compounds as one formula because I wouldn't have found these research documents
as easily. I don't' see any issues with the solution becoming acidic during the reaction so that's a good thing.
*Edit 7/25:
I am still wondering about the reduction process during hydrothermal synthesis. The one document stated a chemical which is a reducer, but can be
explosive if not pure. I've had enough of explosive compounds recently, so I'm not pursuing this route.
[Edited on 25-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I performed the first test on the autoclave today. I added 20mL of distilled water into the 50mL vessel and heated in the oven at approx 180 Celsius
for 6 hours. I'll be opening it tomorrow to measure any mass loss. Hopefully it stayed sealed and had no leaks.

|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
waiting for results
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Leak test was successful. I then performed the test for making FeS2 the next day. I have some pics I need to resize before I can post. I am also going
to setup my laptop to connect to the microscope mounted camera to get better pics. The visual light spectrum won't be enough to confirm the creation
of iron disulfide, so I am still doing my research on testing the end product.
This first test was about as crude as I could possibly imagine, but I do plan on doing some serious refining of my reagents before the next test. I
also have more sulfide material to explore before I get to upload anything.
No matter my success or failures, I have and will be doing my best to document all observations. I'm hoping to see more amateur chemists delve into
hydrothermal reactions. If we can get a good set of data comprised together, there will be a benefit to safety and procedure towards successful
results. I don't feel qualified to be leading anything in chemistry, especially with hydrothermal synthesis, but I like to feel I have something to
contribute from all experience I gain.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
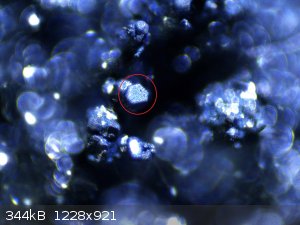
It has a metallic luster under the microscope, but that's just the visual appearance most likely due to the bright LED light and sulfur
contamination.--this was just a test 1st time ever run and I'm not expecting gold colored FeS2. It looks nearly black as shown on the filter paper
and glass slide. I've tested it and it is not magnetic, doesn't dissolve in HCl, solution stays clear nor does it produce H2S.
I'm going to get some sodium sulfite or make some myself as I've been doing with nearly all my reagents. This will assist in removing the excess
sulfur.
 
[Edited on 16-8-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
The lighter brown colored stuff in the black product is mainly iron (III) oxide. I used a nail inside the reaction vessel as a nucleation site, which
was not necessary at all...
There are some really black clumps which had no visual indications of iron (III) oxide contamination. I reserved this stuff for my tests.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
So here's the thing I don't get. According to what little I know of thermodynamics, even 20 mL of water in a 50 mL vessel at 180 C should generate
immense pressures - far beyond the 3 MPa specified for your pressure vessel.
As a rule of thumb, pressure in an enclosed vessel of water increases by about 100 psi (or 0.6895 MPa) for every 1 degree Fahrenheit of increased
temperature. In this case, an increase of 160 degrees Celsius from a room temperature of 20 C would lead to a corresponding increase in pressure of
about 198.57 MPa. (This assumes a closed vessel, but I'm unsure how much of a difference that makes).
Using Barlow's Formula and the specifications for the pressure vessel you bought, a vessel that is 2" wide as an outer diameter would supposedly need
to be 0.9" thick on all sides to prevent bursting at this pressure (leaving you with a measly 0.2" of inner area with which to grow things). However,
a vessel only meant to hold back 3 MPa of pressure would only need to be 0.02 inches thick on all sides, meaning this vessel is likely correctly rated
as being able to withstand this.
At a very rough guess, the thickness of the walls appears to be something like 0.25", meaning this vessel could in theory withstand about 54 MPa of
pressure before it ruptured - but then why rate it so low? And why, in theory at least, would this vessel still burst in the conditions you tested?
I'm not questioning your results, but I am interested in this because I hope to design my own hydrothermal reactor one day, and am wondering if I'm
overengineering it.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | So here's the thing I don't get. According to what little I know of thermodynamics, even 20 mL of water in a 50 mL vessel at 180 C should generate
immense pressures - far beyond the 3 MPa specified for your pressure vessel.
|
I think its about 10bar or 1MPa at 180C
https://www.engineeringtoolbox.com/saturated-steam-propertie...
SodaStream bottles of CO2 are about a 1/4inwall thickness with a diameter about 2.5in from memory are tested at 30bar.

Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I'd considered the steam tables, but I'm not sure if this setup qualifies as 'saturated steam.' Still, your math checks out while mine doesn't - and
those SodaStream bottles have a pretty impressive safety factor of about 3, according to Barlow's Formula at least.
By that metric, though, a hydrothermal quartz-growing vessel operating at a temperature gradient of 355 to 385 oC only generates a pressure
of 17.57 to 23.66 MPa (or 176 to 237 bar), when this process is usually stated to take place at pressures of 700 to 1,500 bar. Why is this discrepancy
so large? The quartz-growing vessels are typically filled to about 80% capacity - would the air trapped in the system somehow generate the additional
pressure, or are these vessels actually externally pressurized and I just haven't seen mention of it?
Apologies for derailing the topic a bit, by the way. I'm just glad to see I'm not the only one thinking about hydrothermal growth!
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | I'd considered the steam tables, but I'm not sure if this setup qualifies as 'saturated steam.' Still, your math checks out while mine doesn't - and
those SodaStream bottles have a pretty impressive safety factor of about 3, according to Barlow's Formula at least.
By that metric, though, a hydrothermal quartz-growing vessel operating at a temperature gradient of 355 to 385 oC only generates a pressure
of 17.57 to 23.66 MPa (or 176 to 237 bar), when this process is usually stated to take place at pressures of 700 to 1,500 bar. Why is this discrepancy
so large? The quartz-growing vessels are typically filled to about 80% capacity - would the air trapped in the system somehow generate the additional
pressure, or are these vessels actually externally pressurized and I just haven't seen mention of it?
|
I do not know how the water vapour could not be in equilibrium with liquid water.
The pressure of a fixed volume gas is approximately proportional to its absolute temperature .
So assuming the gas space volume doen not change then in going from say 300k (room temperature) to 600K (327) the pressure will only double (2bar)
relatively minor increase relative to the water vapour pressure..
However the water also expands and therefore reduces the gas space volume with temperature but it also dissolves in the water.
One way to think about it is: given the density of air at RT is about 1000x less than water, the extra molecules introduced by the air relative to the
number of water molecules is less than 1%. I therefore suspect the air has a insignificant effect on the total pressure at elevated temperatures when
there are many more water molecules than air.
I checked and your correct NaOH method requires about 1200 to 1600bar from the reference I found. So I do not know why it so high. If you assume
1500bar is required that 50x the test pressure of the CO2 bottle mentioned which assuming proportionality and the same material would need a 50x
thicker bottle, 12.5in thick !!! I need to check the test pressure figure and the thickness.
My metal cutting band saw is dull from trying to cut up stainless steel and MOTs so I have not cut open one of those cylinders yet. I will get a
tungsten carbide blade eventually but they are expensive.
[Edited on 19-8-2018 by wg48]
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
element c1: I found a note on Google Scholar that states:
“When this project was started in 1946, it quickly became cvident that the most promising approach mas with a hydrothermal process similar to that
employed by both Spezia and Nacken. Spezia n-orked with temperatures and pressures well below the critical point of water, whereas Sacken found it
advantageous to work close to this critical point. Much time was spent, by numerous investigators, in speculating on the role of gaseous water as a
solvent for such solids as soda and silica. and as a transport medium for silica.“
and
“Kennedy (4) contributed valuable information showing that the solubility of silica in gaseous water is an inverse function of the specific volume
of the gas. This led the author to operate at pressures on the order of 1000 atmospheres at 400" C”
The critical point of water is about 647 K (374 °C) and 22.064 MPa (218 bar) so apparently its possible to operate well above the critical point of
water and well below it., say 100 bar relatively (compared 1500 bar) easily achieved even with a home made autoclave.
I have not fully read the note yet here it is:
Attachment: qgrow.pdf (1kB)
This file has been downloaded 438 times
I suggest any home made autoclave is tested at twice the operating pressure with cold water and only ever used in a hole in the ground not on your
bench.
That attachment failed to open for me try this link https://sci-hub.tw/https://pubs.acs.org/doi/abs/10.1021/ie50...
[Edited on 19-8-2018 by wg48]
[Edited on 19-8-2018 by wg48]
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Yeah, I was definitely thinking of remote operation.
From what I've read, the trade-off for lower pressure is mainly growth rate: the higher-pressure methods using NaOH can allow for a growth rate of up
to 1mm/day in the z-axis direction (apparently substantially less in the x and y directions due to the orientation of the growth plates). I found this paper discussing the specific relationships between pressure and quartz growth rates, and it also mentions that the solubility of silica is
very limited in low-pressure regimes, limiting mass transport (and therefore growth rate) even further. However, this paper doesn't test any pressure
below 1500 bar/150 MPa, and runs the various tests on just 3g of starting material for 192 hours - at maximum, that's a mass transport rate of 0.375
g/day.
I'm still unclear on whether external pressure is needed, but the paper you referenced seems to indicate that they aren't, according to these quotes:
| Quote: | The pressure chamber was filled to 80% of its free space with the
alkaline growing solution at room temperature. On expanding to the operating temperature, the solution density could not be
less than about 0.8 that at room temperature.
Under these conditions quartz crystals were grown at rates
up to 0.1 inch per day, in 1N sodium carbonate solution. |
| Quote: | When the chamber is filled to 80% of its free space
with the alkaline solution, the pressures are somewhat higher with
the higher temperatures, estimated at about 18,000 instead of
15,000 pounds per square inch, as reported previously. |
Even so, this doesn't explain why certain authors can work with varying pressures at the same temperature - nobody seems to mention what, if any,
pressurization methods they use.
My current plan is to build something similar to the apparatus described in this paper - fairly large, with multiple growth sites and a welded
construction - and see if I can't at least replicate some of their results. The main problem is sourcing such a huge steel pipe, not to mention
threading it - I looked into starting with a solid steel cylinder, but I can't even find those past 6 inches in diameter...
[Edited on 8/19/2018 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by elementcollector1  |
| Quote: | The pressure chamber was filled to 80% of its free space with the
alkaline growing solution at room temperature. On expanding to the operating temperature, the solution density could not be
less than about 0.8 that at room temperature.
Under these conditions quartz crystals were grown at rates
up to 0.1 inch per day, in 1N sodium carbonate solution. |
| Quote: | When the chamber is filled to 80% of its free space
with the alkaline solution, the pressures are somewhat higher with
the higher temperatures, estimated at about 18,000 instead of
15,000 pounds per square inch, as reported previously. |
Even so, this doesn't explain why certain authors can work with varying pressures at the same temperature - nobody seems to mention what, if any,
pressurization methods they use.
|
Since the pressure/temperature given is above the critical point of water, the pressure at a given temperature will depend on how much water is
initially loaded into the reactor. The water is present as a supercritical fluid.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
If I recall correctly, they never mentioned the fill rate for any of their experiments, so you're quite likely to be correct. Is there a way to
calculate how much pressure is generated, given, say an 80% fill of the tube at the start?
@Doped-Al2O3-fusion: Have you considered getting a specimen of iron pyrite for comparison under the microscope?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Below the critical temperature and pressure if liquid water is present in equilibrium with its vapour the pressure is determined by the vapour
pressure of the liquid (the steam tables) + the pressure of any other gases present, a small effect if the vapour pressure of the liquid much larger
than the vapour pressure, which is the case above say 10bar and 180C.
Above the critical point of temperature and pressure the water is a gas with a density similar to typical liquids (super critical fluid). Meaning the
pressure is a function of the temperature and the mass per unit of the volume of the container. Just like any gas it expands to fill the chamber..
Near and above the critical point because the molecules are so close together the effects due to the size of the water molecules and the forces
between them are significant so the gas does not obey the usual gas laws, its very no linear. So to calculate the pressure you would have to use an
equation more complicated than the usual gas laws. However you can get a good estimate from the following graph.
If you follow a horizontal from the 800kg/m^3 (that’s the 80% fill) you can estimate the pressure for a given temperature as the line crosses the
pressure curves. Note the graph does not include the effect of the head space air (a small effect) or the effect of dissolved compounds (probably a
small effect as it mostly water)
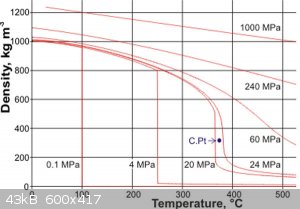
From: http://www1.lsbu.ac.uk/water/water_density.html
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
| Pages:
1
2 |