| Pages:
1
2 |
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
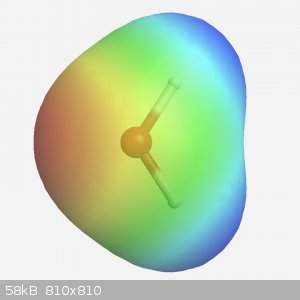
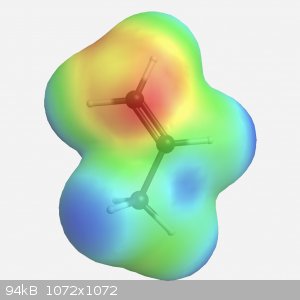
Yeah that’s pretty much it, on a simple molecule (so not a benzene core with multiple subbstituents) you can interpret the electron density quite
clearly. Atoms with lone pairs, so nitrogen and oxygen at the least, are red and lots of hydrogen atoms will show blue. The isosurface is basically
just a graph in 3D space, the best simple description really.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Chaps that this some the most useful information ever! Really fascinating, i expect it plays a bigger role than am i really aware of. But it certainly
makes other organic questions drop into place.
Things like, why would a reaction favor X path and Not Y path. When you see it with clouds, you kind of see why that bit is the bit that sticks on.
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
Opioid chemistry is rather interesting. I am somewhat less familiar with the neurobiological aspects of the drugs as I am with dopaminegic
substances/pathways/neurophysiological actions etc. but it's cool nonetheless. The orientation of the molecule really needs a rotating 3D model to do
it justice i think...
I've always really like the fact that codeine, and diacetylmorphine are effectively in active in themselves as they are both metabolised to morphine
which acts on the opioid receptors directly. Other groups of opioids have similar groupings among themselves... I believe codeine is to morphine what
dihydrocodeine is to hydromorphone (don't quote me on that...)
Hyperbole be thy name
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WangleSpong5000  | Opioid chemistry is rather interesting. I am somewhat less familiar with the neurobiological aspects of the drugs as I am with dopaminegic
substances/pathways/neurophysiological actions etc. but it's cool nonetheless. The orientation of the molecule really needs a rotating 3D model to do
it justice i think...
I've always really like the fact that codeine, and diacetylmorphine are effectively in active in themselves as they are both metabolised to morphine
which acts on the opioid receptors directly. Other groups of opioids have similar groupings among themselves... I believe codeine is to morphine what
dihydrocodeine is to hydromorphone (don't quote me on that...) |
So this then begs the question.....What about methodone? Or however its spelt, if its used for getting people off Morphine, then it has to be both
similar (so it takes some the withdrawal off) and different.
Otherwise little point taking it (apart from purity). Yes its a real shame we are talking about drugs, but honestly its easy to relate to and it gives
alot of information.
Its also got many derivatives, so I am guessing its a great way to learn different mechanisms from one start point. Bloody shame its illegal to mess
with chemically speaking.
When I wasnt so well, I had at various times the following....
Tramadol
Which I dont actually know anything at all about, it was only two weeks I had it for. I dont think its opium based, buts its horrible to take.
Paracetamol with codeine in
. cant remember the name of this. I had it right at the start of when i first started getting pain, crap for pain... No better than paracetamol which
is actually a pretty good painkiller.
Then it all gets a bit odd upto my operation, but Diamorphine, morphine, codeine, oxycontin and one i really cant remember well, it begins with a P I
think and was used just after the operation.
Most were opiates, most were done and dusted in a 4-5 month period. All were different in many ways, so you have one main plant...
The Poppy, not sure what the resin actually contains (apart from the obvious), from that different reactions give you a different molecule. But all
have a similar bit, all I presume have different reasons for existing.
Really interesting and extremely good for explaining OC. Plant alkaloids are absolutely fascinating, the little caterpillar that eats rhubarb leaves,
so it them becomes red banded and toxic for predators. I dont know the Moth its from, but I am aware it can also feed on nettle, but dosnt then get
the red banding.
The ones I really would like to study, are the natural root bearing herbicide ones. R.ponticum is such a menace here, they pay you alot of money to
remove it from your land.
We dont have much on ours, a small patch is left now. We have been ripping it up since we got here! But one thing that fascinates me is the herbicide
it produces in the roots.
You find other plants dont grow well near it, or very well for a year or two after its been removed. Then you get laurel, thats also similar action
wise.
We had a patch of R.ponticum near the laurel trees, both tend to not do well near each other. My interest is to find a tree huggers weed killer!
Weed killer are getting pretty restricted, you need a license to buy it £350 (training course), then if you want to use them in a back pack thats
another £500 on top!
I prefer to find natural solutions to natural problems, i am not so much an environmental type of guy, its just not logical to my thinking, to use
chemicals on soil to kill plants.
Makes more sense to use something from the environment to take care of it.... Alot like Bio control methods for pests, except we often really fuck
that up!
Any other legal drugs or chemicals, that have many derivatives but come from the same start point?
I know some anti depressants are based on plant extracts. What is it in Hops that makes you sleep? I know it used to be a problem for oaste house
sweepers to keep awake during work.
I have also used dried Hops in a pillow to help me sleep.
Sorry gone off a tangent!
Slightly back on target....
Anyone got any idea why the poppy produces morphine? What specifically does the plant produce it for? I have always assumed a predator or maybe a way
to aid seed dispersal.
But I dont actually know WHY, the plant makes it.
The other thing is pine resin, having loads of different spruces and pines around here, means I have tried alot of the resins on bacterial cultures.
Most are excellent antimicrobials , but then again thats what the tree uses it for. Not all pines kill the same bacteria, this is also something I
find fascinating.
Sorry if the drug discussion is a bit disturbing, but honestly from a noobs view point, its a great way to learn about reaction mechanisms.
[Edited on 17-2-2018 by NEMO-Chemistry]
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
Never apologise for your thirst for knowledge. No subject is off lifts and intelligent conversation is a rare commodity these days. The fact that
you're a young bloke is doubly impressive. Drugs can teach us a lot about the brain and if I'm honest, give us an insight into the human condition.
You are comple biological machine whose mind is sometimes subject to it's physical natures... but you also shape the physical functioning of said
machine. Are we the masters or the slaves? Or both... or neither....
Drugs have taught us a great deal about the cogs in the machine so to speak...
Paracetamol (an extremely dangerous substance) is often combined with codeine in OTC (not in OZ... law changed 2 weeks back) preparations or the other
alternative is a double strength codeine tablet on prescription only. I was addicted to codeine for years as I have had counless dental problems (all
porcelain now lol)... I would eat a packet a day of 40 15mg codeine pills. That's 20grams of paracetamol a day. You will die of liver failure within a
week at these doses. Easily rectified with a cold water extraction... para is easy to remove.
Diamorphine is Herion. Diacetylmorphine is its full name (not IUPAC obviously) morphine treated with acetic anhydride yields heroin. I believe it
crosses the blood brain barrier much quicker due to the acetyl groups hence it reaches the brain quicker and gets metabolised to morphine within the
brain where it acts as a major antagonist of the main opioid receptors. I think there are 3 main ones and 14 odd known ones.
All these dugs are Opiates (not Opioids) as the are all derived from the poppy. Oxicodone is a semi synthetic powerful opioid receptor agonist. I
believe they use the morphinon shape to build upon with semi synths... there are loads of esters of morphine as you could imagine.
The Pdrug you took was likely pethedine. Very similar to morphine but without the contipation. This is why it's the hospital go to drug. It has a
small increase in seizure risks.... trade off I guess.
I would conjecture that the poppy make what it makes as it was highly advantageous to those that did. Perhaps a mutation that ended up being highly
advantageous and eveloution (for everything) did the rest.
I've got waaaaaaaaay more to add so bear with me. Sorry my knowledge of opiates is pretty average to be honest. I know phenethlamines a lot better....
Hyperbole be thy name
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I didnt think of plain old selection! Because some poppies dont produce any/hardly any, my assumption was the heroin one has always been potent.
But I guess not, I couldnt come up with a natural reason a plant would make it. Pethedine is correct, I dont remember that much around that time.
The other one that springs to mind is the anti shit yourself pill, Immoden or whatever its called. That also uses small amounts of opiates to stamp
the brakes on, so to speak.
Paracetamol is used alot now in hospitals for pain control, they use the IV version. Depending who you ask, you get reasons from Morphine is hard to
get, to its a safer and better pain killer.
I knew it was hepa toxic, most people assume its harmless because its OTC. Aspirin works really well with me, but stomach problems mean I cant use it.
Which family is Laudnum (i am positive thats spelled wrong!) from?
I have a very old bottle of it in a doctors bag. I have no idea how old it is (bottle is empty), but many the things in the bag are pretty strange. It
includes copper sulphate! Not sure why a doctor would had used that.
I also have a bottle from around the 1930's of Morphine and Kaolin, apparently for stomach upsets! Toothache is one the worse pains ever, closely
followed by ear ache. I regularly get ear ache from my mum, drives me insane 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Laudanum, aka 'tincture of opium' says wiki.
I recall that lettuce can produce something like this.
aha. found it. Search wiki for 'Lactucarium'
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Well thats a bit of a head scratch-er! Wonder how catnip interacts with it (chemically). Astounding what the plant world has within.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Probably gets the cat off it's nips 
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
LOL
Ok i better explain one, i guess not everyone is going to read the whole wiki thing.
Apparently catnip and another plant enhance the effects the lettuce that aga mentioned. I looked at the molecules, but cant see how they interact, but
interact they do.
BTW @google kewls
The lettuce stuff is shit for what your searching for, its great for learning OC, no so great for what your after.
Actually catnip is another plant, what is it about the plant that sends cats into one?
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
That would be the nepetalactones that the catnip produces, so called ‘cat attractants’ and don’t have any effect on humans since they bind to
special olfactory receptors (good luck finding an evolutionary explanation, something to do with pheromones?). It would be hard to say how it makes a
cat feel past euphoric and relaxed, or even stimulated, there are a wide variety of effects one may observe - I’ve personally seen some cats become
heavily sedated and dribbly whereas others become extremely excited, almost manic.
There are plenty of organic molecules that act on our bodies in ways we don’t know for certain, even the UK’s most beloved OTC painkiller
paracetamol, so I have no doubts it could potentially have some modulatory effect. If it does exhibit some interactions in the human body, it
wouldn’t be in the same way as with cats; undoubtedly, some stupid kids have probably smoked some thinking it would be like cannabis, but if it did
get people high then it wouldn’t be so freely available - I could imagine that the experience would, on the whole, be quite unpleasant.
[Edited on 18-2-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
| Pages:
1
2 |