| Pages:
1
2 |
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Strange drawing request
Hi
Ok I am aware this is a really strange sort of request, I have tried to do it myself, I cant work the software, i cant get it right!!!
Could someone do me a 3D drawing in vector format? I need the Oxycodone drug molecule drawn, not as the normal type of way but like the 3D ball and
stick type drawing.
Dosnt matter what angle the final vector is at, the drawing is to form the base of a logo. The rest is not a problem but I have tried to get a vesctor
in ball and stick mode and cant do it!!
i also found images but they are copy write, if you want credit for the logo, i dont have a problem with that. Its for a good cause I can promise
that.
Oh yes the bit where I am told about drug companies getting upset etc, not my problem. Not my website, nothing to do with me or swim or anyone else.
Just I shot my mouth off about my skillz in Adobe, only to find they dont translate to drawing molecules. So while I will fess up I didnt do that
part, i would like to finish what i said I could do.
thx chaps
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I may be completely misunderstanding what you are asking for, but does this work? It is a file from Wikimedia Commons that I took into inkscape and
did a bitmap trace with seventy five scans.
Attachment: Oxycodone_molecule_ball.svg (2.6MB)
This file has been downloaded 672 times
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hmm.
Report or refer OP to chemsketch.
mmmm.
Maybe best to just tell OP to NOT promise something they cannot deliver.
... especially with opiods.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Hmm.
Report or refer OP to chemsketch.
mmmm.
Maybe best to just tell OP to NOT promise something they cannot deliver.
... especially with opiods. |
LOL
Thanks aga, I finally got AI to open it. Now I did want a transparent image background  . But considering I got no where and you quickly delivered....... . But considering I got no where and you quickly delivered.......
I have tried chem sketch, isnt that pay for software though?
I think it might be the one I cant get running on windoes 10 with a AMD chip in the machine.
Anyway thank you alot!!
Yeah my mouth runaway, but its actually for a good cause. I will brush my AI ninja skillz up. i will engage brain and think of wikipedia etc!!
Anyway cheers, now I just got to do the pretty bit.
Inkscape, thats one I havnt heard of. We get a monthly Adobe plan from school. I got the student version that runs out in July.
On linux I use Gimp, But inkscape I havnt heard of, I will give it a spin.
[Edited on 7-2-2018 by NEMO-Chemistry]
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Chemsketch is good. You do not need to pay for the basic installation.
Avogadro is also good. Not as powerful but it does a better job of showing double bonds.
or you could just use the wikipedia image.
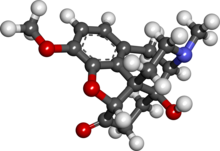
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
WOO how come you lot can get those from wiki?
WTF I am doing wrong or not doing? I will try and install chemsketch again, if anyone got it on windows 10, is there anything I need to do on the set
up to get it to work on windows 10??
Bloody awful OS
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hints and Tips:-
Drink Less booze than aga to Achieve everything you Desire.
Drink More if all you want is spontaneous combustion.
|
|
|
Sulaiman
International Hazard
    
Posts: 3697
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Most molecular structures are available via ChemSpider
e.g. http://www.chemspider.com/Chemical-Structure.4447649.html?ri...
click on the 3D and magnifier buttons below the model.
Click and drag the model in 3D.
Enter any other compound in the search bar .....
EDIT: WARNING ... you may spend too many hours looking at molecules 
[Edited on 8-2-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
LOL it didnt help the J mol thing wasnt working in firefox. I have updated firefox and a couple plugins, so maybe it was that with wiki. i dont let
firefox self update as it breaks alot of things!!
Chemsketch I have never had much luck with it. Chemspider I think must have used something like J mol? It works now but it wasnt working.
I also have a hatred for windows 10, i got used to 8.1, most said it was crap, but I got it going ok. I preferred win7, xp was a security nightmare
but ok system, win7 was IMHO really good. 8.1 worked fine for me, it had some oddball bits but was ok.
Windows 10...... To me its like vista, spawn of the devil.
I prefer Linux but so much of what I use day to day is windows only, wine dosnt solve my problems.
But i do have a linux laptop, I like linux alot. I dont like the commercial almost shareware system creeping in however!
|
|
|
Sulaiman
International Hazard
    
Posts: 3697
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
After clicking on '3D', above the model you can choose Jmol or Jsmol,
depending on your computer, one ot the other or both or neither will work 
P.S. most Wikipedia chemical articles have a link in the panels on the right.
[Edited on 8-2-2018 by Sulaiman]
P.P.S. Once displayed properly you can rotate the model in 3D by click-drag.
[Edited on 8-2-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | After clicking on '3D', above the model you can choose Jmol or Jsmol,
depending on your computer, one ot the other or both or neither will work 
P.S. most Wikipedia chemical articles have a link in the panels on the right.
[Edited on 8-2-2018 by Sulaiman] |
Thx
yes they show up now, not sure why or what setting was wrong... I started with win 10 beta ages ago, this is the last upgrade they did from the free
exchange version. might be time to take it off
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
I can confirm this as being a side-effect.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
I’ve got this app on my iPhone called WebMO. Great for drawing 3D models in a pinch and you can access the electrostatic potential patterns too,
I’ll share both images (let me know if you need a different angle). Also, you can look up pretty much any molecule and then edit as you please,
I’ve used it countless times. You can change the size of the atoms and the bonds, as well as the isosurface parameters, and the background colour
too.
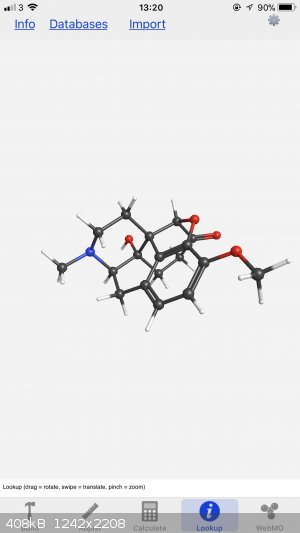 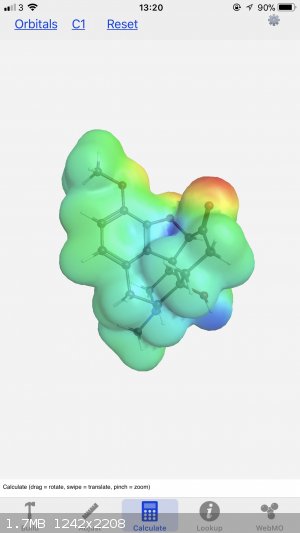
[Edited on 9-2-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That looks awesome LA. I might have to try that.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Yeah they look cool. I looked up morphine and codeine, seeing as codeine is made from Morphine, i figured they must have some the same bonds.
If i got right then they do infact share the same center bit, just different bits branching off. Now this opens up a whole other world!!
Makes OC easier if you can see the molecule, especially if you can see a molecule then one thats related. It makes thinking of groups much much
easier.
The bloody zig zag lines got confusing, i much prefer to see all the bits rather than a cryptic hieroglyph
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
The free version is somewhat limited (basically just small molecules) but you can still access most functions, I paid £5 for the premium but it
really opens up what you can do.
And yes NEMO, codeine is just the 3-methyl ether of morphine, attached to the same oxygen atom as in oxycodone. It acts a prodrug to the latter where
it is demethylated in the liver at about a 5-15% conversion rate. The bit I find interesting is that a lot of opioids actually have completely
different structures, some are based on the morphinan core whereas others like methadone and fentanyl tend to branch off a single atom, which is
diversity not commonly seen in other drug classes.
I find that one of the more useful aspects of the app is when figuring out where substituents will go around benzene atoms - sticking on a group or
two and calculating the electron densities shows how they are altered from the base molecule. Here you can see the difference between aniline and
salicylic acid:
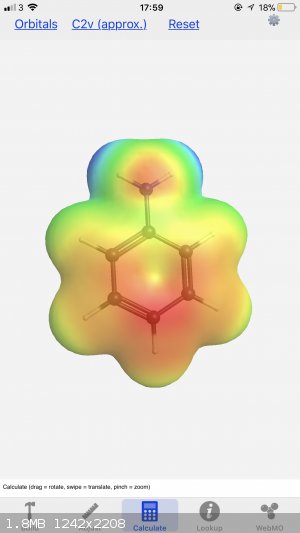 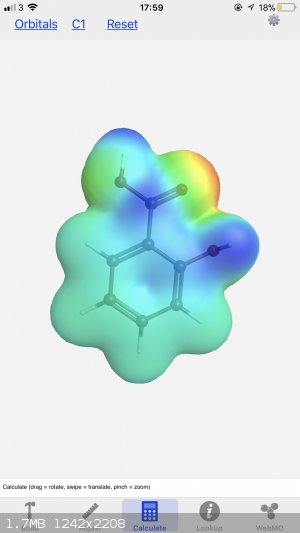
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I didnt make the connection with Oxycodone!! I was aware its synthetic?? So didnt even look at the structure compared to morphine!
Can we find a plant based chemical that legal to discuss this? I am okay with talking about Morphine etc, but i would prefer if we can find a legal
alternative!!! learning organic chemistry via drugs is kinda 'wrong' lol.
Problem is its actually a great example!
I will look the others you mention, from there I can then see how groups are moved around or taken off. Its way easier to understand in that context.
I got stick and balls on order since before christmas, they still not here from China!
I know nothing about analine, so its harder to relate. What about paracetamol and aspirin? I know you can snyth paracetamol from aspirin, but what
about going the other way?
[Edited on 10-2-2018 by NEMO-Chemistry]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
The ‘convential’ opioids (some like to refer to these as opiates as a loose definition) are based on naturally extracted morphine. Most of the
common opioid painkillers are just small tweaks to the morphine molecule, but oxycodone is a bit further away, having a ketone, saturated alkene,
methyl ether (same as in codeine) and an additional hydroxy group.
Actually I found that studying the molecular structures of biochemical and pharmaceutical compounds (legal and illicit, the distinction can get
blurred very quickly but the chemistry doesn’t) is what really got me interested in organic chemistry after the basic stuff in school. Never really
viewed theoretical chemistry as a taboo in any way, even with stuff like explosives and highly poisonous chemicals - it’s when that information is
applied in practice that the problems arise. It’s not like we’re showing people how to make them or anything, or even providing information that
wouldn’t be found with a quick search on Google, I guess that’s the main reason why I feel quite relaxed discussing any chemistry in which I’m
at least somewhat knowledgeable, even if I haven’t or have no intention of carrying it out in practice.
I suck at explaining how these sorts of things work or how to use it, it’s just one of those things you need to have a play around with to get
familiar with, plus it’s not like you have to pay for anything if it’s useless from the start. I’ve been tempted to get some Molymod kits to be
honest, but downloading an app that does the same thing and has a lot more functionality kinda replaced that. Still would be cool to have a few
molecules sitting around the lab though.
Oh, I just grabbed the first two examples I thought of, where NH2 is an electron donating group and both OH and COOH are electron withdrawing. Charts
are good for the basic rules but having the isosurface there really paints a better picture of what the electrons are doing. I don’t think you can
readily synthesise one from the other, not in just a couple of steps anyway - paracetamol is usually made by the n-acetylisation of 4-aminophenol,
whereas aspirin is made by o-acetylating salicylic acid, both with acetic anhydride.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LearnedAmateur  | The ‘convential’ opioids (some like to refer to these as opiates as a loose definition) are based on naturally extracted morphine. Most of the
common opioid painkillers are just small tweaks to the morphine molecule, but oxycodone is a bit further away, having a ketone, saturated alkene,
methyl ether (same as in codeine) and an additional hydroxy group.
Actually I found that studying the molecular structures of biochemical and pharmaceutical compounds (legal and illicit, the distinction can get
blurred very quickly but the chemistry doesn’t) is what really got me interested in organic chemistry after the basic stuff in school. Never really
viewed theoretical chemistry as a taboo in any way, even with stuff like explosives and highly poisonous chemicals - it’s when that information is
applied in practice that the problems arise. It’s not like we’re showing people how to make them or anything, or even providing information that
wouldn’t be found with a quick search on Google, I guess that’s the main reason why I feel quite relaxed discussing any chemistry in which I’m
at least somewhat knowledgeable, even if I haven’t or have no intention of carrying it out in practice.
I suck at explaining how these sorts of things work or how to use it, it’s just one of those things you need to have a play around with to get
familiar with, plus it’s not like you have to pay for anything if it’s useless from the start. I’ve been tempted to get some Molymod kits to be
honest, but downloading an app that does the same thing and has a lot more functionality kinda replaced that. Still would be cool to have a few
molecules sitting around the lab though.
Oh, I just grabbed the first two examples I thought of, where NH2 is an electron donating group and both OH and COOH are electron withdrawing. Charts
are good for the basic rules but having the isosurface there really paints a better picture of what the electrons are doing. I don’t think you can
readily synthesise one from the other, not in just a couple of steps anyway - paracetamol is usually made by the n-acetylisation of 4-aminophenol,
whereas aspirin is made by o-acetylating salicylic acid, both with acetic anhydride.
|
OO
The temptation to look clever was great! Fact is unless i seen a Chemplayer video, i wouldnt know. They took aspirin and went all the way to
paracetamol. Alot of steps I think, I dont remember much except i filed it under might be useful one day.
Not sure for what however!
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
It would probably go something like: ester hydrolysis to get salicylic acid, decarboxylation to phenol, nitration to give O- and P-aminophenol,
purification of the latter, reduction to give p-aminophenol then react with AA with the OH group protected (TMSCl, benzoate?) or for lower yields, in
neutral/acidic medium. I guess a more readily accessible way for the last part would be the neutralisation with acetic acid then dehydration to the
amide, then you shouldn’t have problems with the OH getting in the way.
[Edited on 11-2-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It was NileRed that did this. I think ultimately 7 or 8 steps and from memory similar to what you have described, LA. Yield was down below 1% for
the whole process -- some steps were very low yield.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Sorry Nile!
I dont remember much about it, at the time I was just watching OChem videos to try and get a handle on it. Part of the problem is I am lazy by nature,
and O chem is really not for the lazy!
But its some of the most interesting chemistry (after bio chem obviously  ) )
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The Lazy tend to make a lot of Sound and never any Action.
The old addage "empty vessels make the most sound" has a poignant meaning with amateur chemists.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
So what are the 'clouds' showing in the drawing? Is this the most active regions electron wise or bonds flexing, or whatever you want to call it. AND
can we [please get a roll eyes smiley, or better yet a rolls eyes grumpy?
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
The "clouds" are what clouds always are in chemistry - an approximation of electron density. I say approximation because in theory the elextrons
extend to infinity inall directions. The surface shaded represents a zone with a certain probability of containing the electrons and represents a
reasonable im age of thed shape of the molecule at least inasmuch as molecules interact with one another.
The coloured diagram gives more information. The colours indicate electrostatic potential - that is, the force that a point charge would experience if
it was situated in that position. As such it indicates positively and negatively charged regions in the molecule which is useful for figuring out
reaction mechanisms as well as intermolecular forces.
|
|
|
| Pages:
1
2 |