| Pages:
1
2 |
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Let me send you a piece of tellurium.
I'll send a u2u.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Radium Clock Safety
I am two weeks out from my next stocking of the display, and I have acquired several new samples since my last post. One of which is a vintage radium
travel clock which I bought before thinking about the safety of such a sample.
From my research, the paint contains primarily Ra-226 which decays by alpha emission with a 3% chance of gamma emission, but also may contain other
transuranic elements that are prone to beta decay. I am not concerned about alpha radiation and given the long half-life of Ra-226, the gamma
radiation is probably not much higher than background radiation. My concern is beta radiation. Most places I found say that beta particles can be
stopped by plastic or wood, but I cannot find any exact numbers on how thick the plastic or wood needs to be. Is 5.5 mm of acrylic enough to stop the
beta radiation or do I need a secondary shield?
I am thinking that the best thing to do is to put the clock in an acrylic box and put the acrylic box in the display. What do you think? Am I
concerned about nothing?

Oh, and thank you to j_sum1 for the beautiful tellurium.
[Edited on 23-9-2017 by Plunkett]
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
You can use a thin layer of glass to stop the beta radiation if you are truly worried.
The philosophy of one century is the common sense of the next.
|
|
|
CaCl2
Harmless

Posts: 39
Registered: 14-1-2017
Member Is Offline
Mood: No Mood
|
|
For gold you could have some colloids, they are pretty and interesting.
https://en.wikipedia.org/wiki/File:Gold255.jpg
The problem is that aquatic "solutions" of them are always at least somewhat unstable.
Maybe the best sample would be if you managed to obtain a piece of authentic "cranberry glass", in which the gold nanoparticles are suspended in the
glass, coloring it red.
It isn't really made anymore, and a lot of what is sold as cranberry glass is actually colored by selenium, etc.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I have some I would like to donate.
What are you still missing?
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I have added to the display a rough diamond, an ampoule of sodium, a polished disc of silicon, potassium, nickel, germanium, tellurium, two light
bulbs with nice visible tungsten filaments, and a small piece of gadolinium. I also have grey arsenic and the radium clock, but I am not putting them
in until I can seal the arsenic in an ampoule cast in resin and verify the activity of the radium clock with a Geiger counter. I only put the clock
in the display long enough to take a picture.
The diamond looks more like a granite pebble, but it is in fact a diamond. The beautiful ampoule of sodium was bought from user Chemcraft and the
tellurium was donated by j_sum1. The nickel pieces are “nickel trees” that form when there is a hole in the insulation in the tanks used to plate
car bumpers. The trees slowly grow on the exposed surface until a worker notices them and breaks them off. Normally they are sold as scrap but the
company Flex-N-Gate was nice enough to send me a few. The silicon and germanium pieces are unfinished optics for infrared imaging systems and were
donated by the company Lattice Materials.
Of the fist sized chunk of lithium, the technetium lead pig, the xenon short arc-lamp, and many other wonderful samples I have had the fortune of
handling, I have to say the neatest one for me is by far germanium. Sure, it is not super interesting to look at, but I got to hold a three-inch puck
of solid germanium. Who else gets to do that? It is twice as dense as silicon and feels unlike any other element. It feels and looks almost like a
metal but it is a little to hard and a little too shiny. I sat for a good ten minutes just holding it and turning it in my hands.
Enough of that, here are some pictures. I also went back and took better pictures of all of the samples which can be found here
         
Below is an updated graphic of the progress of the display. I am going use the rest of the grant to buy most of the lanthanides, phosphorus, and some
heavy water or tritium. I plan on making boron and maybe cesium over winter break, and I am going to find some pretty rocks for francium and radon
unless somebody else has a better idea. The rest of the samples are up in the air, and I plan on handing off responsibility for the display to
someone else who is still at the high school come summer time.
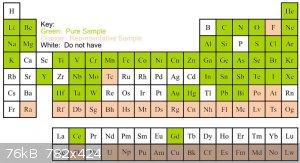
I have downloaded the SDSs for all of the elements and compounds in the display onto a flash drive which the school will have in case of an emergency.
I want to make physical copies, but the SDSs total over 600 pages. If I printed only the remotely hazardous ones it would be half that, but that is
still a lot of paper. I am reluctant to print them, but physical copies would be more accessible if something were to ever happen. What do you think
I should do?
An interesting thing has happened to the iodine sample. When I first put the sample in the display all of the iodine was loose in the bottom of the
ampoule, but now crystals cover the walls of the ampoule. I think what is happening is when the light is on it heats the iodine, sublimating a
greater portion than normally would, and when the light turns off and the sample cools, the extra iodine vapor iodine deposits on the walls of the
ampoule. It is purely aesthetic, but it is something to consider if you plan on displaying iodine in a similar fashion.

Lastly, here is a nice photograph of all of the salts I had or was able to make. From left to right there is Iron(III) oxide-hydroxide, Cr2O3, CuO,
Fe2O3, KMNO4, CoCl2, MnCl2, K2CrO4, TiCl3, MnO2, and a number of colorless salts.

|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
This has been a cool project and executed really well. I hope you feel pleased with what you have accomplished. Just lovely.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I ampouled the arsenic today. I still have to cast it in resin before I put it in the display but the dangerous part is over. Some members have the
experience and facilities to work with heavy metals like arsenic, but I do not. All I did was use tweezers to move pieces of arsenic from one
container to another, and it was one of the scariest things I have ever done.
Up to this point, the most dangerous chemical I have worked with was bromine. I am fine with bromine; it fumes profusely, burns your lungs, and eats
through gloves but at least it is clear what you are working with. However, arsenic is just another dull grey metalloid; nothing about it screams I
will kill you ten times over. I am 99.9% sure my safety precautions were adequate, but that 0.1% is the stuff of nightmares.
Maybe I am overreacting, maybe not, but I thought I would share my experience and a photo of the sample. The ampoule is 18 mm wide.

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
This is amazing work. Very well done!!
I think the iodine sample looks wonderful. The little sparkles of purple in the photo are beautiful, and I bet it really glitters in
person. I also have some arsenic that I should ampoule, and that will be equally scary I'm sure. Ampouling bromine was one of the scariest things I've
done; the thought of accidentally knocking it over while the top was still hot, leading to instant boiling and possible explosion of the glass was
horrifying. Made it through safe though!
Keep up the great work. I'm really enjoying watching the progress on this project.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Thats the kind of project that would crowd fund well. Excellent work, its a legacy that will live on long after you have forgotten it. Superb stuff, I
am pleased your school got behind you, after all they have gained something very unique.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
The display is finally finished. Yesterday I put in the last set of samples, and hot glued every other sample down because they were shifting from
the vibrations caused by a nearby door opening and closing countless times over the course of a year. Except for fluorine, vanadium, and rhodium, all
the stable element samples are >90% pure; the gas ampoules contain the respective gases at significantly above atmospheric concentrations (except
for nitrogen); and yes, that is a bite taken out of the indium sample.
For the radioactive elements, I contacted my state’s Department of Health Radiation Division and they said that given the levels of radiation
involved, and average exposure time, the samples pose no significant harm. I thought as much, but now I have it in writing from a government
official. Radon, francium, actinium, and protactinium are represented by pictures of the decay chains for U-235, U-238, Th-232, and Am-241, all of
which are found in the display. I did order some uranium ore for a radon sample, but I forgot to buy a vial to put it in, so I put the ore in the
uranium box instead. Given how hard promethium is to come by so I decided to make my own representative sample from an old temperature gauge and
United Nuclear’s europium based UltraGlow phosphorescent paint to show how promethium is used.
All the rare earths were bought as a set from Metallium. They came in labeled vials, but they were not the clearest, so I took the air stable ones
out of the vials. To prevent mixing the samples up, I engraved the element symbol on the bottom of each sample with a Dremel tool and a diamond burr,
because except for dendritic samarium, they all look and feel the exact same. Their sameness is a little boring, but it demonstrates an important
point about the similarity of the elements and their presence in the display shows students that the lanthanides are not just an extra row at the
bottom of the periodic table that nobody cares about, which is the impression I got taking high school chemistry.
The most expensive sample per gram was rubidium at $30 for 15 mg. The most expensive sample I purchased was iridium at $68 for a 1 g pellet, and the
most expensive sample donated by a company was germanium which I estimate at over $100 based on eBay prices. Not counting my time and samples donated
by companies, the total cost was $3000, however there are a few things I can think of to make the display cheaper if I did it again. This project was
fun, but it took far longer than I thought. I want to build another display for my university, but that is a far-flung dream for now.
As before, photos of the rest of the samples can be found here. For whatever reason, the photos I took this time round are not as sharp as the earlier ones.
          
|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Just beautiful!
Actually, I am surprised that you accomplished this so quickly and so cheaply. It really is a mammoth undertaking and you have done really well.
Your radium sample is identical to mine.  Ditto sodium. (Thanks Chemcraft!) Ditto sodium. (Thanks Chemcraft!)
I think you have done a great job of Al and that Ti sample is really quite cool and dramatic. I am still flicking through tthe pics.
|
|
|
| Pages:
1
2 |