| Pages:
1
2 |
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Periodic Table Display for my High School
Today I have completed a massive project spanning half a year and hundreds of hours of work, a periodic table display for my high school. It all
started last summer when I was at Griffith Observatory in California where I saw they a periodic table display with sizable samples of each element. I was not new to element collecting, having started my own earlier that year, but
my collection consisted of dinky ampoules and this display took up a whole room. Needless to say, I wanted my own.
In my musings on the matter, I came across Theodore Gray's periodic table table and periodictable.co’s displays but the former seemed beyond my skillset and the latter prohibitively expensive. My dream was slowly dying, but
then I found a blog by a Nigel Coutts, a teacher who had built a periodic table display for his school. I was a high school student, I had a school, maybe
this could work. Loosely following Coutts lead, I drew up plans and presented them to a few teachers who all wanted to see this idea become a
reality. I submitted a request for funding to my school’s parents and friends foundation which was subsequently approved. I now had $2500 to work
with and I more or less knew how I was going to build the display so I started work. I did not know what I was getting myself into.
I will spare you the details (ask if you want), but let’s just say that I am very familiar with multiples of 118. 59 acrylic panels to laser cut,
118 holes to drill, 236 trim pieces to glue, 354 connections to solder, ad nauseam. I had planned to do most of the work over my spring break but the
project drug on and only just today did I put the finishing touches on the display, 3 months overdue. In the end, there are 118 boxes (130 mm x 130
mm x 127 mm) enclosed by laser cut panels of acrylic bearing the name, symbol, and atomic number of each element and lit with a 2 W LED.
In total, it cost about $2000 to build the display, leaving me with $500 for samples. That sounds like a lot, but as many of you know elements
aren’t cheap. I am going to try to get the display in the local news as part of a fundraiser for more samples. As it stands, I have pictures
representing the super heavy elements, a button of Americium 241 from a smoke detector, and samples lined up for aluminum, sulfur, bromine, chlorine,
iron, copper, lithium, fluorine (fluorite), and polonium (sparkplug).
 
  
I am not going to ask for handouts, but I am asking for help. I really want to make this display something that catches student's attention and helps
teachers teach otherwise mundane lessons in an interesting way, but appealing samples are prohibitively expensive. Many of you have skillsets, labs,
and contacts far beyond my reach. For instance, I know there are people on this forum skilled at making alkali metal ampoules that are shiny and free
from oxidation, or glassblowers that can make discharge tubes. I can pay for the materials and shipping if you are willing to provide your services.
Even if you do not have any special skillsets, if you work at a place where there is scrap titanium, or know who I can contact to get factory reject
silicon boules, etc., I would be glad to hear from you.
In addition to elements in their pure form, I am also looking for colored transition metal salts to teach students about oxidation states. I only
need about 2 or 3 grams of each salt to fill a small vial (see picture) that I will put in the box with the respective sample. I already have iron
(II), iron (III), copper (II), manganese (IV), manganese (VII), and chromium (III).

Lastly, if you have any other ideas about how I could use this display as a teaching aid (lessons, demonstrations, etc.) I am all ears.
[Edited on 15-6-2017 by Plunkett]
|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Very cool.
I think I can help -- with ideas if not samples.
I will write more later.
J.
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
500 goes nowhere with elements of any great sample size unfortunately, especially if you want high purity.
[Edited on 15-6-2017 by diddi]
Beginning construction of periodic table display
|
|
|
Texium
Administrator
       
Posts: 4675
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
I might be able to send you small samples of some metals and salts. I'll look through what I have available and send you a message when I get a
chance.
|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have to agree with diddi -- $500 is small fry for display elements. You probably won't get too far with that.
But a lot depends o exactly what you want for the collection. Do you necessarily require the item in the elemental state? Do you need a visual
sample of the element or do you just need to showcase its use? Does it matter if it is impure? Are you restricting yourself to one representative
sample for each element? (Carbon could be fun to choose in that case.) You might want to consider how authentic you need to be -- particularly with
gases. Do you actually care if an ampoule of colourless gas is air or neon? I have seen ampoules for sale that possibly once contained elemental
fluorine mixed with helium but now are pretty much nothing.
You mentioned showing the different oxidation states of transition metals. For that, I would recommend solutions in test tubes rather than dry
compounds. A lot easier to put together and ultimately a whole lot less expensive. This site will give a good starter for that.
If you were going for relatively cheap options then I would recommend samples that showcase the element's use. Elemental form where possible but not
exclusively. Theo Gray has a lot of lovely photos that are not all elemental but are all interesting.
One thing to consider is that you might put a stand-in in your display and replace it when something better comes along.
Here are some thoughts on some of the elements:
H -- tritium key-ring fobs are available for not too much. You might think of a way of showing all three isotopes.
He -- go to a party balloon shop and see if you can get something representative -- a small labelled canister of ballo0n gas would be ideal but a
mylar balloon might be ok.
Li -- an intact lithium AA battery along side a clear jar of mineral oil containing the foil extracted from the battery.
Be -- if you can't get a metallic sample, go for a piece of beryl
B -- fun and easy to make via thermite. MrHomeScientist has a straightforward YT video showing the procedure
C -- What to choose? Maybe a large lump of coal. Simple but it says a whole lot.
N --
O -- gases present their own difficulties. Ideally a gas discharge tube that shows the spectrum but these things aint cheap.
F -- a roll of teflon tape is the easiest. Or if you can obtain a piece of antozonite that would be good. Failing that, a fluoride salt.
Ne -- small neon bulbs are available.
Na -- I just bought a nice ampoule of shiny Na for my collection. Not amazingly cheap but worth it. But a sample stored under mineral oil should be
reasonably doable.
Mg -- There is a recent thread on this. Mg ribbon is easy to find on eBay. Likewise Mg ingots. You might obtain a Mg firestarter or pencil
sharpener or a section of a mag wheel.
Al -- easy. The trick is finding something interesting. For your display do you know someone who could sculpt something intriguing out of kitchen
foil?
Si -- Not too difficult to find some on eBay or to make it yourself via thermite. But for a display such as yours I would go for a section of Si
wafer.
P -- Ideally a full set of allotropes in matching ampoules. But not many element collectors have that. An artistically arranged matchbox could work
though.
Cl -- This is just coloured enough to be visible in an ampoule if you can get it. Liquid would be even better.
Ar -- Like neon, little light bulbs are available. You might want to think of how they could be lit up.
K -- A sample under mineral oil should be possible and really takes a bit of beating. Ampoule is even better. Plastic banana as a last resort.
Sc -- good luck.
Ti -- Powder is cheap and easy to get. Rod would be nice. Or get a manufactured component -- a bolt or a climbing equipment or some overpriced
cutlery for adventure enthusiasts
V -- Vacuum deposited dendrites can be found but are not cheap. You could display a chrome-vanadium wrench. V is a good transition metal for showing
oxidation states.
Cr -- you should be able to find something chrome plated
I won't do all all of them, but here are some other considerations
Au -- gold leaf is easy to get and displays well. Other foils are also available.
Pt -- you might dissect an old catalytic converter and display it
In -- is used in touchscreens if you can't get an actual element sample.
Ba -- used as a getter in old valves
W -- filament of an old light bulb
Hg -- tilt switches are easy to get
Th -- found in thoriated tungsten rods
At -- send me a U2U. I have a brilliant idea for my own collection. I'll spill the beans if you ask.
You have not mentioned where you live -- that will have an impact on anyone sending stuff to you. It will also impact what is available for you -- it
seems that Ga and Bi are both a lot easier to get in the US than they are in Aus for example.
Hope this helps.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I can probably help you out. I have a sample of low-grade uranium ore, that will set off a geiger counter but just barely. They prospected the area
during the 30s or so, and decided the ore wasn't of sufficient quality to mine.
Iodine, just get a bottle for $10 from China on eBay.
Indium and gallium, I have substantial amounts of the pure metals. I can sell at bulk price, which is about 50 cents a gram. Gallium is used in the
highest-efficiency solar panels in the form of gallium arsenide (as is arsenic). But they're expensive, so they're mostly used for satellites.
However, quite a few LEDs use gallium arsenide too, typically the infrared ones that are used in TV remote controls, so that might be a good example
product. Ruthenium, I can also offer for cheap, in a gray powdered form. I have vanadium pentoxide, an orange powder, which I'd throw in for free.
For carbon, I can probably get you a mediocre-quality diamond for free as well. I also have boron carbide, an industrial abrasive. Thorium, you
should probably get from a lantern mantle, as the thoriated tungsten rods are 98% tungsten, and as a result look like that's what they're made from.
Not very interesting either, since they look like metal rods. Tin is used in lead-free solder, I can send some of that for free too. There is (or at
least used to be) a small piece of beryllium oxide in microwave ovens. It shouldn't be hard to get it out if you can find one. However, it's toxic,
so a better example might be an emerald, which has beryllium as one of its constituent elements. (Synthetic is fine, and much, much cheaper)
I've made manganese before by reducing manganese oxalate (prepared from potassium permanganate and a lot of oxalic acid) with aluminum, then
dissolving the rest of the aluminum away with sodium hydroxide. It's not super impressive. A dull, dark gray metal.
You can get element samples for the ones that are more annoying to acquire on eBay, they're often pretty cheap:
http://www.ebay.com/sch/i.html?_nkw=element+sample
As for salts, I can get you chromium VI, probably all the vanadium ones (or at least the most common ones) in solution, palladium II (not actually
super expensive, since it's very strongly colored so you only need a little bit), silver nitrate (kind of boring, it's white), ruthenium VI, as sodium
or potassium ruthenate. Possibly selenium; I've been meaning to get some anyway.
Neodynium should be easy, the magnets it's made of are in everything.
Actually, for transition metals, you should really use solutions when possible, rather than salts. Potassium permanganate solutions are brilliant
purple, but the salts look kind of boring. Sort of a dull, purplish gray. And if anyone makes these salts, they're likely going to get them in
aqueous solution anyway. Additionally, some salts like sodium ruthenate are not isolatable in a solid form, and others, like palladium chloride,
would be prohibitively expensive to fill a vial with, but 25 mg can easily color 10 mL of water. Also, I don't think it's that important if the
anhydrous form is a different color than the hydrated form, if they're in the same oxidation state. It's just confuse high school kids.
[Edited on 6/15/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I know $500 is not a lot to work with, but it is what I have. I am not trying to fill the display all at once, rather trying to stock it with a
quality starter set that can be filled in over the years. Unfortunately, with the way the school works, I can only spend this money at certain
vendors (Amazon, United Nuclear, Flinn Scientific, etc.), but I have plans for a fundraiser later in July and whatever I get there will not be bound
by these restrictions. As such, I am not going to start buying samples until I know how much money I have to work with.
For storing reactive metals I do not have access to an argon tank, but I do have grocery store mineral oil. I have heard that you can boil the
mineral oil to reduce the amount of dissolved oxygen, is this true? And, would I do good to buy some sacrificial sodium to dry the mineral oil? For
less reactive metals like copper and zinc do you think I can get away with a coat of clear spray paint or fingernail polish?
| Quote: | Originally posted by j_sum1
You have not mentioned where you live -- that will have an impact on anyone sending stuff to you. It will also impact what is available for you -- it
seems that Ga and Bi are both a lot easier to get in the US than they are in Aus for example.
|
I live in Arkansas, United States.
| Quote: | Originally posted by j_sum1
Do you necessarily require the item in the elemental state? Do you need a visual sample of the element or do you just need to showcase its use? Does
it matter if it is impure? Are you restricting yourself to one representative sample for each element?... Do you actually care if an ampoule of
colourless gas is air or neon? |
I would like for the item to be in its elemental state, but really I am happy with whatever captures students' attention and plays into a lesson. For
instance, I have a piece of fluorite for fluorine and I can talk about how it is so reactive that nothing can contain it. Or, with platinum, I will
have a piece of a catalytic converter because it is visually more interesting than an a few centimeters of a wire that could be anything, and I can
use it as a starting point for a discussion on catalysis. But, if a sample is reasonable to obtain in its elemental state, like beryllium, I want to
get an actual piece of metal, not just a compound stand in.
I do not care if a sample is impure so long as it is not misleading. For example, for tungsten I will probably get a bucking bar that is ~80%
tungsten.
I am not restricting myself to once representative sample for each element. For carbon, I am thinking a nice piece of anthracite coal, a stick of
graphite, and a diamond (or cubic zirconia stand in). Phosphorus will be fun too.
For the gases, I would like to get discharge tubes, but until then I will have ampoules filled with normal air, and the light bulbs I bought when my
local RadioShack went out of business.
| Quote: | Originally posted by Melgar
Iodine, just get a bottle for $10 from China on eBay.
|
I have seen mixed opinions on this forum about iodine and phosphorus from ebay, and living in the US, I really do not want to get my door kicked in.
Have you purchased iodine from ebay without problems? Because I am working with a high school I can buy from Flinn Scientific, which has 100g of
iodine for $40, and cross my fingers maybe from Sigma Aldrich, which has 250g of Red P for $40. If nothing else, I know my school has some potassium
iodide I can make iodine from.
| Quote: | Originally posted by j_sum1
You mentioned showing the different oxidation states of transition metals. For that, I would recommend solutions in test tubes rather than dry
compounds. A lot easier to put together and ultimately a whole lot less expensive. This site will give a good starter for that.
|
I think that is a great idea to use solutions rather than dry compounds. I was thinking in my world of pottery dyes that are a few bucks a kilo not
tens of dollars a gram. I am not trying for all of the metals and their oxidation states, just the first row and a few others here and there to
demonstrate the point.
My current list of element ideas so far:
H – tritium vial, ampoule of air, heavy water if the budget permits
Li – I have a 50g chunk
Be – an x-ray window or gyroscope sphere
B – I have plans for boron thermite, but I dread filing the magnesium
C – Anthracite coal, stick of graphite, diamond (or cubic zirconia)
F – I have a piece of green fluorite (CaF2)
Ne – Light bulb
Mg – I have a vapor deposition crystal
Al – I have a piece of extruded aluminum tubing with an interesting cross section
Si – I want a boule/wafer but I will probably get polycrystalline chunks from my school
S – I have a puck
Cl – I have the requisite chemicals, could I make a large ampoule out of a volumetric flask?
Ti – overpriced outdoor cutlery or a part of an airplane
Fe – I have a block of iron
Cu – I am going to grow crystals with electrolysis
Zn – I have a few dollars of post-1983 pennies I will melt
Ga – I think the lights in the display will melt the gallium, so any ideas on how to present molten gallium?
Br – I have enough NaBr to make two liters of bromine, how much is too much? I am thinking a large ampoule with a small amount of bromine at the
bottom and a headspace for the vapors
Kr – Light bulb
Mo – Midwest Tungsten has some Mo pieces in their orphan bin
Ag – US Silver Eagle Coin
In – Get an ounce or two and hammer it into a cookie, then take a bite out of it to demonstrate its softness
Sn – I am going to make a think acrylic box, fill it with stannous chloride, then electrolyze it to grow tin crystals
I – Flinn Scientific or Potassium Iodide
Xe – Xenon flash tube
Cs – I have some CsCl and some lithium to make cesium with
W – Tungsten bucking bar
Pt – Catalytic converter
Au – Gold leaf
Hg – I have a mercury contactor I am going to take apart
Pb – Scuba dive belt weight
Bi – Bismuth crystal
Po – Firestone Polonium Sparkplug
Rd – Radium Watch Hands
Th – 2% thorium filament from a microwave magnetron
U – Uranium ore, fiestaware, or depleted uranium if I have the budget
Am – smoke detector button
[Edited on 15-6-2017 by Plunkett]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Plunkett  |
For storing reactive metals I do not have access to an argon tank, but I do have grocery store mineral oil. I have heard that you can boil the
mineral oil to reduce the amount of dissolved oxygen, is this true? And, would I do good to buy some sacrificial sodium to dry the mineral oil? For
less reactive metals like copper and zinc do you think I can get away with a coat of clear spray paint or fingernail polish? |
You can heat the oil to drive off gases, but boiling it may be a challenge as this requires upwards of 300C. United Nuclear sells 'low oxygen' mineral
oil already that you could use instead of pharmacy brand. If the container is sealed well enough, whatever you put in it will react away residual
oxygen and water, corrode a tiny bit, then be inert from then on.
Also the 'less reactive' metals don't really need anything done to them. I've had copper and zinc on my element display for years and they don't look
much different.
I get that invisible gases aren't very interesting and nobody will know the difference, but is it not 'misleading' to put up ampoules of air as real
element samples? Or a cubic zirconia as a real diamond? I think it's dishonest and does a disservice to the students if you do that. You went through
all this trouble to build an awesome element display, so fill it with real elements!
Quote: Originally posted by Plunkett  |
I have seen mixed opinions on this forum about iodine and phosphorus from ebay, and living in the US, I really do not want to get my door kicked in.
Have you purchased iodine from ebay without problems? |
I've bought iodine from eBay with no issues. If you buy it from Flinn and have it shipped to your school there's no way that will raise red flags of
any sort. Or as you said, just make it from potassium iodide easily enough.
| Quote: | Originally posted by j_sum1
You mentioned showing the different oxidation states of transition metals. For that, I would recommend solutions in test tubes rather than dry
compounds. |
My only concern with this is stability of the solutions. Some things may be just fine over time, but others might not. I know I tried storing
potassium permanganate solution and that eventually degraded into brown MnO<sub>2</sub>. Just something to consider; research before you
commit to it.
Finally some comments on your list, in red:
Quote: Originally posted by Plunkett  |
My current list of element ideas so far:
...
B – I have plans for boron thermite, but I dread filing the magnesium
Buy Mg powder from eBay or United Nuclear! That's what I did.
Cl – I have the requisite chemicals, could I make a large ampoule out of a volumetric flask?
That would look awesome, if you can seal it well enough. A regular ampoule on its side might also work, so you can
look down the length of the tube and see the color better.
Ga – I think the lights in the display will melt the gallium, so any ideas on how to present molten gallium?
Are those LEDs really that hot? If they do melt it, be aware that molten Ga wets glass. I have mine displayed as
little beads (made by dripping molten Ga into water) in a sealed ampoule, and luckily it hasn't had any temperature 'accidents'.
Sn – I am going to make a think acrylic box, fill it with stannous chloride, then electrolyze it to grow tin crystals
Sounds awesome, but in my experience those crystals are very fragile. Not sure how they'd survive over
time.
Cs – I have some CsCl and some lithium to make cesium with
Have you researched how to do this? Did you read Dan Vizine's thread about making it here on SM? It's not a trivial
task to make an element that explodes on contact with air.
Au – Gold leaf
Precious metal foils are great for displays because they are cheap and show off the metal brilliantly. You should be
able to get foils in many of these metals that would look good on your shelf. |
Finally finally, a question about your display. How do you get the elements in there? Does the back or front open up? I'm working on my own Periodic
Table Display 2.0 to replace my current ones, and there's a few design choices like that which I need to make.
Great job on the cabinet! It looks professionally done.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yes, buying iodine from China will work, and is arguably not even illegal, since the laws apply to the sellers, who aren't under US jurisdiction.
Possession of them CAN be illegal, but police have to prove intent. The only reason it's even illegal in the first place is because if the police go
through your garbage and find iodine-containing waste but little else, they have probable cause to ransack your house. If they'd actually wanted to
put meth cooks out of business, they would have made pseudoephedrine prescription-only, but that would put a bunch of "meth task force" officers out
of work. So instead they made two out of twelve of the reactive non-metals on the periodic table illegal to buy or sell in their elemental forms.
However, if you'd rather avoid that whole ball of wax altogether, just get Lugol's solution from Amazon. It has elemental iodine in it, costs about
$10 for two bottles, and is perfectly legal.
Of course, if you're having trouble finding any element in particular, this place can sell you virtually all of them:
http://onyxmet.com/?route=information/periodic_table
For gallium, get a Geratherm Mercury-Free Thermometer. They're like $5 at Walmart and similar on Amazon, and contain a gallium alloy instead of
mercury.
Small amounts of bromine in a large ampoule are pretty cool, because they can function as a thermometer. The hotter it is, the more orange you see.
Also, it's kind of crazy watching orange-black drops condensing on the sides.
For zinc, get one of those die-cast conduit connectors from a hardware store. Like this:

Zinc is often used to make stuff that needs to be harder than plastic, and easily cast into complex shapes, so another common use for zinc is in
locks, for the tumbler.
Diamonds are actually surprisingly cheap if you get a non-gemstone-quality rough. Check it out:
http://www.ebay.com/sch/i.html?_nkw=rough%20diamond
The kids will learn what most rough diamonds look like too!
For storing reactive metals, a sealed vial is usually enough, for everything except maybe the alkalis. For those that are really reactive, just get
one of those spray air cans that have tetrafluoroethane in them, spray some in the vial, then drop in your sample and tighten down the lid. It's just
as unreactive as anything else you could use at room temperatures, and is the same thing as R134a, if you happen to have any of that around.
For oxygen, you can use hydrogen peroxide, which gives off oxygen bubbles and is 36/38 oxygen by mass. Also, a little globe, and a figurine of a
person, since both the earth and people are mostly oxygen. I've always been fascinated by the Oxygen Catastrophe, but that might be a little much for
a periodic table display. Earth, incidentally, is the only place in the universe that we know of that is predominantly an oxidizing, rather than a
reducing environment. It's just that due to a number of factors, like the CNO cycle, oxygen's affinity to metals, etc. our planet ended up with a
disproportionate amount of oxygen relative to the rest of the universe. And even then, our planet used to be a reducing environment until microbes
started using light to separate all that useless oxygen from the elements that they needed.
[Edited on 6/15/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
You haven't mentioned most of the transition metals, so I'll help out a bit:
Zn: from pennies! (post-1982) Melt them down and collect the zinc the drips out of the pennies.
Zr: cubic zirconia (as you mentioned)
Nb: apparently some piercing stores have piercings made of niobium, and they're not terribly expensive. INS if they are still Nb though.
Mo: Try to find molybdenite (MoS). It's very shiny but somewhat expensive. Some lubricants use MoS, but that should be a last resort.
Tc: Haha. Maybe an X-ray from a Tc injection?
Ru: Ebay. A bit pricey though. Melgar seems to have offered it, so take his deal.
Rh: Wait for the price to drop (or not). Rh is used in catalytic converters, and sometimes fake jewelry because it's very shiny (shinier than any
element except silver).
Cd: Ni-cad batteries or cadmium sulfide paint is ideal. Cadmium is a pain to deal with due to its high toxicity.
Sb: Old tin toys are often a tin-lead-antimony alloy. Antimony goblets have been used for medicine apparently.
Te: Blueray CDs may have a Te oxide coating, but these are rare. Ebay is best.
Hf: One of those buttons from a plasma torch. Some use hafnium bits (?) to cut because they are durable, but INS if that's true.
Ta: Anything that's electronic uses tantalum capacitors, but good luck finding them.
Re: Ebay. Way too useful in the military (and pricey too).
Os: Old needles and old fountain pen tips used osmium, but nowadays, eBay is better.
Ir: Spark plugs are sometimes iridium. Osmium and iridium are often alloyed together, so it might be misleading.
Bi: My local science museum sells small samples of bismuth at their souvenir shop. Otherwise, maybe extraction from Pepto-Bismol? Or buy on Ebay.
Please correct me if I'm wrong or if these are incorrect.
[Edited on 6-15-2017 by ninhydric1]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Actually Tc isn't that hard to get. Hospitals have this thing called a "moly cow" that contains molybdenum that decays into a metastable form of
either Mo or Tc, I forget which. The metastable form is very useful for imaging, but then decays into the more stable form of Tc that doesn't really
have any practical uses, and has a long half-life. Lots of people that collect elements have Tc from moly cows, which incidentally got their name
from the fact that you have to milk them periodically to get the isotope they produce. If they don't need that isotope for a few days, then they just
have some extra Tc in an isotope without many uses.
As far as rhodium vs. silver, silver is actually less shiny than aluminum by a little. It's shinier than rhodium but tarnishes such that it's less
shiny than rhodium almost immediately. It's easy to get something rhodium plated, which would look pretty much identical to rhodium from the outside.
Any non-chain jeweler should be able to do it.
[Edited on 6/15/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Good luck finding one of those moly cows though. They are pricey too.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  |
I get that invisible gases aren't very interesting and nobody will know the difference, but is it not 'misleading' to put up ampoules of air as real
element samples? Or a cubic zirconia as a real diamond? I think it's dishonest and does a disservice to the students if you do that. You went through
all this trouble to build an awesome element display, so fill it with real elements! |
You are right, I do need at least try for the gases or include a label with % abundance in atmosphere.
Quote: Originally posted by Plunkett  |
My current list of element ideas so far:
...
Ga – I think the lights in the display will melt the gallium, so any ideas on how to present molten gallium?
Are those LEDs really that hot? If they do melt it, be aware that molten Ga wets glass. I have mine displayed as
little beads (made by dripping molten Ga into water) in a sealed ampoule, and luckily it hasn't had any temperature 'accidents'.
In the early stages of design, I did some tests to see how hot the LEDs got because I was worried about burning the
wood and I found that if I run the LEDs at full power (~1.5 A) they can get up to 100 °C. They are meant to be mounted on a heat sink not on wood. I
did further tests in a sealed box at a more modest 750 mA and the temperature in the box rose above 35 °C; Ga melts at 30 °C. In the final product
I have the LEDs running at 500 mA and I have not measured the temperature.
Cs – I have some CsCl and some lithium to make cesium with
Have you researched how to do this? Did you read Dan Vizine's thread about making it here on SM? It's not a trivial
task to make an element that explodes on contact with air.
I have read part of Dan Vizine’s Cesium from CsCl[i/] and a few other sources. I am working on a resistance
furnace with a PID controller that should be able to reach and maintain the requisite temperatures. I bought the CsCl to make Cs for my personal
collection and I will be wearing full safety gear, so worse comes to worst I am a few dollars down the drain with a caustic mess to clean up.
|
Quote: Originally posted by MrHomeScientist  |
Finally, finally, a question about your display. How do you get the elements in there? Does the back or front open up? I'm working on my own Periodic
Table Display 2.0 to replace my current ones, and there's a few design choices like that which I need to make.
|
I did not take many pictures during the construction process, but I do have CAD drawings of the entire display. If you want more details than this
U2U me. There are eight screws that hold the side trim onto the display so it can be removed. Then, there is another set of wooden bars behind the
trim that you remove and then you can slide the acrylic out. The acrylic is a bit undersized so it can slide easily in a track that is formed by the
trim you see and a rabbet cut in the boards that make up the meat of the display. You have to be careful not to scratch the acrylic sliding it out,
but I figured with a hundred students walking by it each day it was going to get scratched anyways. It is inconvenient if you want to access the
middle of the display because you have to slide out all of the panels in that row. There are probably better ways to do it for a home display, but
that is the how I did it.
A note on lighting: I used a forstner bit to drill recess for the LEDs so they would not be shining in people’s eyes; this caused a shadow around
the top of each box. Also, I did not cut a groove for the wiring to sit in and so it also cast a shadow on the back of the box.
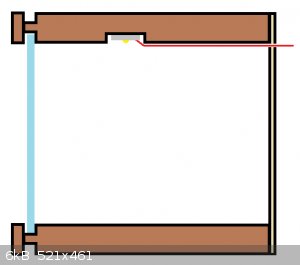
Cross section of one level of the display

Before I put the horizontal trim pieces on
That is a great idea that I will definitely use.
Quote: Originally posted by Melgar  |
Earth, incidentally, is the only place in the universe that we know of that is predominantly an oxidizing, rather than a reducing environment.
|
I will have to include that fact in one of the lessons.
[Edited on 15-6-2017 by Plunkett]
|
|
|
phlogiston
International Hazard
    
Posts: 1381
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Melgar, I doubt any element collector owns a visible sample (by bare eye) of technetium.
I've worked with radioactive materials in a hospital (for research, not treatment or diagnosis), and I suspect that diverting a significant quantity
of it from any western hospital is (fortunately) very difficult. Obviously, a hospital (or any other company/institute) will only receive the permits
to order and handle such materials if they have implemented adequate security measures, personal training, waste management, etc.
Furthermore, because the specific activity of Tc-99m is extremely high (1.92E17 Bq/g), the dose given to a patient in terms of mass is infinitesimally
small, on the order of nanograms. An entire moly cow contains only micrograms of Mo-99.
So, you would need to divert 100's of entire moly cows or process the the pee from millions of patients to obtain milligram quantities of Tc, which to
my standards constitutes the minimum for a visible sample (by bare eye) for an element collection.
I'd love to have a sample of Tc, but I've come to accept that that is very unlikely to happen.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Isn't the daughter product of Tc-99m another isotope of Tc with quite a long half life? If so, a sample is theoretically possible -- although having a
visible quantity will be problematic for reasons you state.
I would be satisfied with an empty bottle or vial or some similar thing labelled with Tc for my element collection. I have not found a source yet
however.
My U2U inbox was flooded with requests about At. (Flooded is a relative term but more than the usual number in the inbox.)
Brilliant idea is probably overstating it. And actually, it is an old idea so does not qualify as novel.
Apparently, some time after At was discovered a quantity was synthesised for study. Bi was bombarded with alpha particles. So, with an alpha source
and some bismuth you could set up a facsimile of the experiment. My idea was to position some bismuth in the end of a test tube (bismuth mirror perhaps) and a smoke detector button in the other. Then seal as an ampoule. Of course the alpha particles are not numerous enough or energetic
enough to really do anything. But there is a remote chance that an at atom will be produced every now an then and the setup will tell the story of
the element quite well.
(Realistically there is likely to be more At in a lump of radioactive ore than the above setup but for a non-visible sample that is pretty much
academic.)
If you wanted to improve on the above you could wrap the test tube in a coil that could, in principle, accelerate the alpha particles to hit the
target more energetically. I doubt it would work without significant engineering and there would be no way of determining its effectiveness. But
again, it tells the story.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by phlogiston  | Melgar, I doubt any element collector owns a visible sample (by bare eye) of technetium.
I've worked with radioactive materials in a hospital (for research, not treatment or diagnosis), and I suspect that diverting a significant quantity
of it from any western hospital is (fortunately) very difficult. Obviously, a hospital (or any other company/institute) will only receive the permits
to order and handle such materials if they have implemented adequate security measures, personal training, waste management, etc.
Furthermore, because the specific activity of Tc-99m is extremely high (1.92E17 Bq/g), the dose given to a patient in terms of mass is infinitesimally
small, on the order of nanograms. An entire moly cow contains only micrograms of Mo-99.
So, you would need to divert 100's of entire moly cows or process the the pee from millions of patients to obtain milligram quantities of Tc, which to
my standards constitutes the minimum for a visible sample (by bare eye) for an element collection.
I'd love to have a sample of Tc, but I've come to accept that that is very unlikely to happen. |
The few people I've met with Tc samples, it was a barely-visible dot (which I highly doubt was even pure Tc), and the only way you knew it was there
was with a geiger counter. Maybe you have to work in that department of a hospital or know somebody who does, but it IS attainable, and since the
radiation is beta, it's not particularly harmful.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I live near a decent size medical research institution. I will get in contact with them and see if I can get an x-ray as ninhydric1 said or a
vial/lead pig that was used to store technetium like what Theo Gray has.
Quote: Originally posted by j_sum1  |
Apparently, some time after At was discovered a quantity was synthesised for study. Bi was bombarded with alpha particles. So, with an alpha source
and some bismuth you could set up a facsimile of the experiment.
|
I like the idea but I think it would go over most people's heads. Maybe it would strike a chord with the physics students.
|
|
|
phlogiston
International Hazard
    
Posts: 1381
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Quote: Originally posted by Melgar  | | The few people I've met with Tc samples, it was a barely-visible dot (which I highly doubt was even pure Tc), and the only way you knew it was there
was with a geiger counter. |
Pretty cool. As you say, Tc is certainly collectible from a safety and stability perspective. Which makes it all the more frustrating that it is
nearly impossible to get.
At least for other difficult elements like thorium, radium, polonium and uranium one can use minerals or items in which they are (or were) used as a
source. Not so for Tc.
This site has a nice collection of elements and pictures of a really nice sample of Tc (not his own):
http://www.periodictable.ru/043Tc/Tc_en.html
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
invisible gases: very interesting. I use plasma discharge to display them. I have a full set of 150mm ampoules all the same size and I zap them!
its awesome. there is also a guy who makes BIG tubes with whatever gas but they are a bit dear for me atm
Beginning construction of periodic table display
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
molybdenum should be relatively easy and fun to get. I have a whole bunch of molybdic acid and using that to make a molybdenum thermite was quite easy
and fun, I posted about it here:
http://www.sciencemadness.org/talk/viewthread.php?tid=28565
If you like I can send you a small amount of molybdic acid so you can make the thermite yourself. It would be a good idea to read through that thread
and incorporate some of the suggestions that blogfast25 added though.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Oscilllator  | molybdenum should be relatively easy and fun to get. I have a whole bunch of molybdic acid and using that to make a molybdenum thermite was quite easy
and fun, I posted about it here:
http://www.sciencemadness.org/talk/viewthread.php?tid=28565
If you like I can send you a small amount of molybdic acid so you can make the thermite yourself. It would be a good idea to read through that thread
and incorporate some of the suggestions that blogfast25 added though. |
Thank you for the offer but I do not have any aluminum powder and my furnace is still a long way from functional. I think for molybdenum I am going
to get a disk or boat from tungsten.com's orphan bin.
Starting tomorrow I will be off the grid on backpacking trip. I should have an update on the display when I return in early August.
[Edited on 17-6-2017 by Plunkett]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Update
I am back from my trip and I have started work on acquiring samples. Currently, I have samples for lithium (I will get a better container), fluorine
(fluorite), carbon (anthracite coal), magnesium (vapor deposition crystal), manganese, iron, cobalt, lead, xenon (flash tube), cerium, mercury,
polonium (sparkplug), and thorium (2% thorium filament from microwave magnetrons). For those of you looking for mercury for your own collection,
mercury displacement relays or mercury contactors are a great source. I got 51 g from one relay that cost me $15 shipped. Opening them up can be
difficult but I found a pipe cutter works well.
I am waiting on samples of sodium, gold, and graphite to arrive in the mail. I plan on making bromine this week and chlorine when the volumetric
flasks I ordered come in. I contacted the medical research institution near me and they gave me a lead pig with a technetium eluate collection vial.
The man who gave it to me said it was only used for training and never had any actual technetium in it, but it is a nice representative sample. I
have also written around thirty letters to different companies asking for samples. So far, three have responded and are sending me unknown quantities
of sulfur, copper, gallium, germanium, indium, tin, and bismuth.
Lastly, for those who have offered me samples, I will get back to you in the next couple of days. I have just been busy between my trip getting ready
for college.

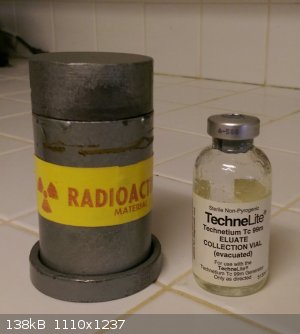
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Great project! One that's especially close to heart for me :- )
I'm happy to help you fill in your table with some freebies. What are you still missing?
Even Tc is doable, though hellatiously pricey
Cheers,
Rasiel
rasiel@luciteria.com
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Update 8/11/2017
Yesterday I put the first set of samples into the display. The display now features forty elements in their pure form and five representative
samples, making the display 60% full. You can see the more interesting samples as well as a color coded chart of the progress below. The rest of the
samples can be found in google drive here. They are a little blurry because I was in a hurry to get home and pack for college. Admittedly some of the samples are a little lame like
the chrome plated ball bearing and ampoules of colorless gases, but others, like copper and iron, I am quite proud of.
              
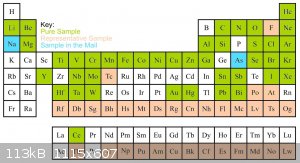
Now some ramblings about my experiences sourcing samples:
I never thought I would get such a great response from companies. I sent out thirty letters hoping that two or three would send me samples. So far I
have received twenty-two samples from ten different companies. Thank you Admat Inc, Advanced Radiation Corporation, All Metals Sales Inc, AMG
Vanadium, Asbury Carbons, GES Graphite, University of Arkansas for Medical Science, Rotometals, Montana Sulphur and Chemical Company, and Georgia Gulf
Sulfur Corporation.
Previously, I said I was going to put 51 g of mercury metal in the display. I had a great plan for how I would contain the mercury with a glass vial
inside of a soda bottle preform that I would then epoxy shut so nobody could open it. But, in the process of sealing the mercury in said container I
managed to spill a goodly portion of it on my workbench. I spent three hours cleaning up the mess, fortunately none made it to the floor, but my
mercury had become contaminated with bits of antimony that were on my workbench and had become dull. The finished sample looked so horrible that I
put it straight into my mercury waste container and never looked back. Fortunately, I had an old thermostat and mercury thermometer which I think
turned out to be a more interesting sample in the end.
I never knew how many different chemicals my high school has. I was given access to the high school’s stockroom to look for samples and there was
an unending trove of reagents ranging from mercuric iodide, to phthalic anhydride, to uranyl nitrate. There were probably over 100 unique compounds,
and not just a few grams of each. There were three unopened one-gallon bottles of nitric acid that had probably been there since the seventies, two
large bottles of galena, and potassium iodate out the wazoo. It is a shame students never get to use any of them. On the bright side, I got
selenium, silicon, tin, and iodine for the display as well as some magnesium powder to make boron with. I also filled up every small vial I had with
different compounds giving me around twenty-five in total.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
What are you planning to do for phosphorus (it isn't marked on your periodic table)? Some of the strike strips for matchboxes and matchbooks contain
phosphorus, so it could be used as a sample for phosphorus (it is a restricted substance).
For tellurium, some Bluray DVDs have a thin layer of tellurium oxide, so you could use a Bluray DVD as a representative sample.
You could probably find a radon test kit. A piece of granite could work too, as granite contains radioactive elements that can decay into radon.
Scandium is found in aluminum alloys that make some high-performance sports equipment, such as lacrosse sticks or baseball bats.
Neodymium magnets are great examples for neodymium.
Terbium is used in some speakers (famously, Soundbug) in the form of terfenol, an alloy containing terbium.
Surprisingly, Himalayan sea salt contains many rare earth metals.
|
|
|
| Pages:
1
2 |
|