| Pages:
1
2 |
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
70g doesnt yield 70x70 times more energy, however larger quantities of energetic material tends to give off energy faster, this is related to critical
diameter, but it isnt anywhere near 10 times as much energy per "time" unless if were talking milligrammes vs tonnes
i would really like to see linoleum peroxide, its what forms when linoleum gets air and the resultant peroxidation which is exothermic heats up, every
now and then resulting in rags with linoleum on 'em catches fire, possibly a weak peroxide
ether peroxide on the other hand, im gonna assume this still havent been made
ap is a gamble, you should with exactly this think more than do
|
|
|
greenlight
National Hazard
   
Posts: 754
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
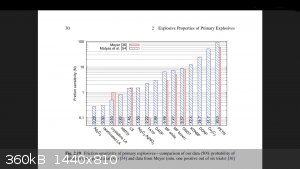
Just thought I would put this up. It's a friction sensitivity results graph for common primaries that has TATP and HMTD results as well.
Most research doesn't bother with peroxides so it is unusual to see them on there.
[Edited on 20-5-2017 by greenlight]
Be good, otherwise be good at it 
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by Booze  | Quote: Originally posted by Tetra  | Quote: Originally posted by Booze  | | Should I be aware of this? I saw one video, and the guy just throws it around and keeps 70 grams of it, and has kept it for 7 months. Then I saw
another video saying you should not keep it for more than 10 hours and be extremely carefull with it. I just made a batch, and it is in the fridge
right now. Is it really that dangerous, or is it something to be laid back about? |
Pardon my French, but I highly recommend that you DO NOT fuck with organic peroxides. Personally, I made over 200g over the course of three years,
without incident. I was extremely lucky, others weren't so. I've found that it's just not a risk I'm ever willing to take again. So, I swore never to
make any more organic peroxides. And I haven't since. Disposing of them is a nightmare. Chemically destroying them is very tricky, and I still haven't
found a method that is reliable. Don't risk it. Be safe.
-T/Azide
[Edited on 12-5-2017 by Tetra] |
I like to make things that go boom. What do you mean disposing of them is a nightmare? I dump most my chemical waste in a bucket and dump it in the
gutter. |
...and that's a terrible idea, as well as a bad thing to do.
[Edited on 22-5-2017 by Tetra]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Booze  | Quote: Originally posted by Tetra  | Quote: Originally posted by Booze  | | Should I be aware of this? I saw one video, and the guy just throws it around and keeps 70 grams of it, and has kept it for 7 months. Then I saw
another video saying you should not keep it for more than 10 hours and be extremely carefull with it. I just made a batch, and it is in the fridge
right now. Is it really that dangerous, or is it something to be laid back about? |
Pardon my French, but I highly recommend that you DO NOT fuck with organic peroxides. Personally, I made over 200g over the course of three years,
without incident. I was extremely lucky, others weren't so. I've found that it's just not a risk I'm ever willing to take again. So, I swore never to
make any more organic peroxides. And I haven't since. Disposing of them is a nightmare. Chemically destroying them is very tricky, and I still haven't
found a method that is reliable. Don't risk it. Be safe.
-T/Azide
[Edited on 12-5-2017 by Tetra] |
I like to make things that go boom. What do you mean disposing of them is a nightmare? I dump most my chemical waste in a bucket and dump it in the
gutter. |
You are one reason home chemistry is in decline, no offence but your attitude needs some adjustment. Fortunately Darwin has a habit of cleaning up
genetic mistakes.
|
|
|
| Pages:
1
2 |