| Pages:
1
2
3
4
5 |
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
It would be expensive, but what about pumping compressed O2 directly into the sulfur burner?
Then having another oxygen inlet going into the reaction tube where pure O2 is pumped in preheated via a torch or whatever?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Air works just fine so why bother ?
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
I was thinking that
a) the sulfur would burn better and at a more easily controlled rate using pure O2... no need for a wick
b) since there is no moisture in the system, cheap iron pipe could be used with little corrosion
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
BeerChloride, you're right. I mistranslated 'pure' as meaning 100%, the book was using the 'pure' for the constant boiling not 100%. But then 100%
acid takes SO3 to get.
Burning in air actually gives more SO3, the small amount of NOx formed catalysis the conversion of SO2 + O2 to SO3, as in the chamber process. Burning
sulfur in pure O2 gives a fairly intense flame, try it on test tube scale sometime. Burns better, but controlled may be problematic
To avoid moisture, dry the air that feeds the burner. Use calcium chloride or silica gel.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Maybe a conversion of the lead chamber process would work, Mix your sulphur with nitrate and ignite by heating it for a while with a hot flame. (When
they're properly mixed you might even be able to with a few sparklers os something similar.) Lead the exaust gasses, (SO2 and NOx) through the pipe
with the catalyst or maybe even just a hot pipe, the NO2 will convert the SO2 to SO3 and the NOx should revert back to nitrogen (not sure though). The
SO3 can be led through H2SO4 to complete the synth.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
When I was fresh out of college I worked at a pulp mill that used CaSO3 as a bleaching agent. The CaSO3 was made on site by first burning sulfur to
SO2. There was a view port to look at the burner. It appeared to be a series of jets that looked just like what you'd see in a furnace burning
natural gas. This makes me think that the sulfur was vaporized prior to burning. Now that seems like it would be controllable and trouble free, if
properly designed.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Sounds like a reiteration of what I've said already. I understand the air needs to be dry, (well actually it doesn't matter if it's SS 316) and have
accounted for it. I guess the thing I'm most interested in is making the sulfur burner. I do not see why a large enough wick wouldn't produce enough
SO2. I guess what I need to do is saturate a piece of kaowool with liquid sulfur, wait til it cools, then mass to see how much sulfur loading there
is. I know that it's about a 2.5:1 factor for a liquid of 1.3 g/mL (experiments with other catalysts on this useful substrate): Per gram of kaowool,
two and one half times that of the liquid will be held.
Nerro's idea is interesting, but it might be hard to control. I'm afraid of over doing it and pushing way too much SO2 through the system and
overheating it.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
It might be controllable by adding amounts of something inert like ground up silica to moderate the reaction. Experimentation with different ratio's
of silica might give you the correct amount to give you a steady but modest flow of exhaust gasses.
You might also spread the gasses through the sulfuric acid more effectively by placing an inert wool like material inside it and passing the bubbles
through that. It will break them up and disperse them while keeping their rate of ascent low.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I may have mentioned this before:
How about a very small jet of O2 impinging on molten sulfur in an atmosphere of sulfur and SO2, followed by an afterburner? Has anyone tried it?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Fleaker I think I understand what you are saying with the wick. I'm not saying this, or any other method, might not work.
I'm just saying that upon reflection of what I think I saw many years ago the sulfur may well have been a vapor before it issued from the jets (which
was probably just a drilled pipe) and was burned.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I think the problem with any wick sort of burner is the
same as what would be the problem with just setting
an open pot of sulfur in the combustion chamber and letting it burn , and that is the temperature at which sulfur is fluid and free flowing will soon
be exceeded by the combustion chamber temperature , causing the
unburned sulfur to gell and thus making the replenishment of sulfur in the closed combustion chamber a problem while it is in operation . So any
design reliant upon molten sulfur in a fluid state as the
fuel feed for the burner is going to require some sort of reservoir which is controlled at a lower temperature
than the combustion chamber temperature in order for the molten sulfur to remain fluid enough and not become too viscous to feed the burner .
The molten sulfur is quite flammable so getting it to burn in the hot combustion chamber is no problem , and it
would likely burn very well simply dripped upon a small
mound of broken " lava rocks " like are used in gas cooking grills , if these were heaped in a clay saucer
like is used under clay flower pots as a drain pan .
Ever noticed how the melted fat burns on those lava rocks when it drips from a steak being grilled ? That
is the idea , and the burning of the fuel keeps the rocks
quite hot enough so that any new molten fuel dripping
upon them is also inflamed and the burning continues ,
with a continuous flame there on the ceramic material
acting as a burner and igniter for fresh material as it
arrives by drips . Increase the drip rate and you get a bigger fire , decrease the drip rate and the burner throttles back to a minimum sustainable
idle level .
So the combustion process is throttleable .
On a molten sulfur reservoir , the temperature will need to be thermostatically controlled , and the drain valve
and the exit tubing also will have to be wrapped with heating tape and thermostatically regulated so that the
sulfur remains at the most fluid temperature and can be flow regulated at a steady viscosity . And where that
feed line enters the much hotter space inside the combustion chamber it must be insulated against any undue heating which would thicken the sulfur and
prevent its flowing from the discharge . Indeed some
flow rate of the feed sulfur will be needed to keep this
last section of the discharge line free flowing and it
should be large enough bore so that it is self draining ,
perhaps even jacketed with oil or something which would
be temperature limiting for that final length inside the combustion chamber above the burner where heat
could complicate the flow of fuel to the burner . This
would be the logical location for the inlet air to the combustion chamber , coaxially flowing inlet air around the molten sulfur delivery tip would
keep it shielded
from the much hotter gasses of the combustion chamber .
Keeping the molten sulfur at the proper temperature
within the last few inches of the feed line is really the
most technically difficult hurdle of the entire scheme ,
but entirely manageable .
The scale of the burner and the rest of the apparatus
should be along the lines that could burn several kilograms of sulfur per day at a minimum , for any
practical amounts of product , otherwise what you
are doing is investing a lot of time and work and materials in a novelty which won't produce any worthwhile amounts in any reasonable time .
A simpler alternative if you want to manually feed sulfur
periodically to the burner is just to cast your molten sulfur
into short cylindrical ingots just slightly smaller than a metal tube which is mounted to deliver the chunks of solid sulfur to an open pan burner in
the combustion chamber . When the
burner gets low on fuel , you simply load some sulfur ingots
into the tube and push them through with a rod , where
they fall into the burner . It may not be high tech ....but it should work like a charm .
[Edited on 28-10-2006 by Rosco Bodine]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Sulfur is cheap, 50lbs would be something like 10 dollars from the local farmer supply. I haven't even bothered to do the stoichiometry yet to see the
maximum (theoretical) SO3 output. I appreciate your advice Rosco, I think what I could do is set up some sort of thermostatically controlled sulfur
melter of perhaps 1 gallon in capacity and use a ball valve to control the flow rate. This would be in an elevated position and would flow down into
the reaction chamber. I like the concept of throttling it down or up, but I still recognize that additional dry air must be supplied to the combustion
chamber. I plan to also use a slightly faster 'jet' of air at the top of the apparatus (near the admittance point for the sulfur drip tube) that would
create a pressure differential in the bottom of the combustion vessel and thereby draw the sulfur dioxide into the reaction tube at a faster rate,
with oxygen at an excess.
Curious, but does anyone have information on catalyst service life (i.e how long before degradation occurs)?
Thanks
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I have been thinking about that open pot sort of burner
using a simple feed of solid ingots for replenishment from
a horizontal side tube , and there is a way to throttle
that sort of burner too by using a disc or a perforated
disc or dome to variably cover or expose the surface of the burning sulfur in the open ( or not so open ) pot .....
if you use the principle of a damper . This sort of scheme
could simplify the design of a prototype combustion chamber considerably and eliminate the neeed for any
thermostatically regulated molten sulfur feed systems .
You know how if you have a fire in the kitchen in a pan of hot oil , you simply put a lid on it to extinguish it ....
and if you had the lid center mounted on the end of a rod
so that you could raise or lower it gradually over the burning pot ....then you could throttle the flames across
a range of intensity . A cover plate could also be pivot mounted so that as it pivots it eclipses the open pot across the range of from fully open to
fully closed .
So there are a couple of ways an open pot burner could be throttled if you follow what I am describing .
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
I realize the going topic here is focused on the SO2 production, but here's some freezing data I compiled on H2SO4:
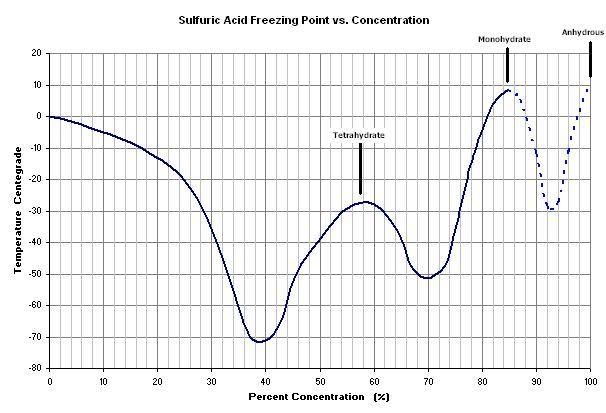
The mono- and tetra- hydrates can be identified in the graph. The dashed portion entails some estimation.
Now I know why my brown 94% didn't freeze. The impurities probably lower it even further (guess - 45 ?). Someone asked where to find dry ice. It's
available at the grocery store here (surprising, I know), but it should be able to be found at food/catering supply establishments. I'm getting rather
curious to try freezing.
Dry ice is -78 C, so it should work. What should I do? Acetone and dry ice? Does acetone simply provide heat transfer, or are there other reasons for
this mixture?
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
BeerChloride, Maybe you should try to dilute you brown H2SO$ a little bit so it is in the 85% range, according to your graph it would be much easier
to freeze out, maybe even with a standard freezer!
Doesnt look like my "battery acid boil down" is going to materialize today...its raining and very windy so I would have to do it in the closed
garage...BAD IDEA!
Mericad
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by mericad193724
BeerChloride, Maybe you should try to dilute you brown H2SO$ a little bit so it is in the 85% range, according to your graph it would be much easier
to freeze out, maybe even with a standard freezer!
Mericad |
That would be the mono-hydrate freezing out, You'll not get any more concentrate acid than that by freezing once you take to the 85% or lower range.
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Yeah, it crossed my mind to dilute it, but that extra concentration above 85% is precious - where the real dehydrating power is. But, it could still
be a way to purify it in order to have some not-so-concentrated stuff. Incidentally, the 94% might be close to eutectic, I don't know, but I don't
expect the freezing to concentrate anything. I'm just aiming to purify and keep the 94% concentration.
I meant to get some dry ice earlier and sublimed some iodine instead...
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
This maybe a really stupid comment but....
Can't you just do electrolysis on a sulfuric acid solution to drive off the water as H2 and O2. This can just be led off into NaOH or outside. This
setup can be run over night and maybe produce 90%+ from 30% acid? Eventually the conc. acid would just act as a short circuit so you should include a
fuse to blow and then you got conc. acid right?
Will this work, I will try if it is a yes...This seems safer than boiling
Mericad
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
You will form sulfurous acid, oxygen(with small % ozone, this is actually a prep for dilute ozone in oxygen gas mixtures), hydrogen(most likely, but
the redox potentials don't completly indicate this), and water.
[Edited on 29-10-2006 by The_Davster]
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Good try, but won't work. The reduction potential is higher for the H+ to be turned into H2, rather than from water. Thus, the acid will be destroyed.
Many electrolytic processes drive the pH up at the cathode, and can be a way to make hydroxides. I saw a thread somewhere in here, though, that may
have mentioned making H2SO4 by electrolysing magnesium sulfate or something.
Just another thought: another possible variation might be to concentrate batt. acid to about 87%, which should freeze out monohydrate around 0 deg.,
the remaining liquid will be more concentrated.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Good try, but won't work. The reduction potential is higher for the H+ to be turned into H2, rather than from water. Thus, the acid will be
destroyed."
What will the acid be converted to?
Turning H+ into H2 (and OH- into O2) is exactly what we want to do here.
As far as I can see this method will work to produce relatively conc acid from dilute stuff (albeit slowly and expensively).
Unless the nascent hydrogen reduces the HSO4- to SO2 I can't see a problem and, since car batteries outgas H2 if they are overcharged, I don't think
that reduction will happen (at least not in solutions as concentrated as battery acid).
What happens in really conc H2SO4 might be different, but I think this should work.
For what it's worth, the same idea (electrolysis) is used to dehydrate HF because most drying agents don't work.
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Well, unionised, I just don't know. What the_Davester said sounds plausible. But you raise a good point about car batteries, and I read that they give
off hydrogen AND oxygen when overcharged due to electrolysis of water. To me it makes sense, though, that the cathode will put electrons into the H+
(properly H30+) ions which will then combine into H2 leaving water. Oh, wait, the concentration dependence! A steady-state equilibrium. For dilute
acid it might work, but at a certain point of H+ concentration, the H2 production balances. That is why the overcharged battery bubbles, and the acid
cannot be concentrated any further. I think the H2 comes from the acid.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
I remember vaguely that I once read about producing H2O2 by electrolysis of sulfuric acid. Perhaps that might also be formed.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
With HF it works because look at the potentials:
F2 +2e- <-->2F- +2.87V
O2 + 4H+ +4e- <--> 2H2O +1.23V *
2H+ +2e- <--> H2 0 *
2H2O +2e- <-->H2 +2OH- -0.83
So When electrolysing HF, you get a net of:
4H+ + 2H2O --->H2 + O2 +4H+, so water is removed as you said.
However with sulfuric...
O2 + 4H+ +4e- <--> 2H2O +1.23V *
SO42- +4H+ +2e- <-->H2SO3 +H2O +0.137 *
2H+ +2e- <--> H2 0
SO42- + 4H2O +6e- <---> S(s) +8OH- -0.751
SO42- + H2O +2e- <---> SO32- + 2OH- -0.936
2H2O + 2e- <--> H2 +2OH- -0.83
So when electrolysing sulfuric:
2SO42- +8H+ +2H2O --> 2H2SO3 +2H2O +4H+ O2
Will aproximatly happen, because the acidic sulfate is a better oxidizing agent than H+, then what happens with HF will not happen here. But, based
on the number of preps for preparing everything from ozone to oxone from sulfuric electrolysis, a lot of other products may form depending on the
conditions, but one thing is certain, that the water will not just be electrolysed out.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Last time I checked the electrolysis of dilute sulphuric acid to give H2 and O2 was a standard high school demonstration (as well as a common
observation with overcharged batteries).
If you tell me that it doesn't work, because the ionisation potentials say so, then I say you have got something wrong.
The minor technicality that you are quoting standard oxidation potentials whereas the real reaction we are talking about is miles from the standard
conditions is one point that may explain this.
More to the point, if the H+ is removed from the acid or the water it doesn't matter- the mixture will re equilibrate. If the system loses H2 and O2
then the overall effect is loss of water.
You also seem to have come up with a cell reaction that doesn't make sense- peroxide oxidises sulphite rapidly so they can't both be the products of a
real reaction.
Since, as has been pointed out, ozone and H2O2 are minor products the overall mixture will be oxidising- S(IV), if formed, has a fair chance of being
reoxidised to S(VI).
Peroxydisulphate is another reasonable product, but it's not much of a problem it breaks down to H2SO4 and "O"
|
|
|
| Pages:
1
2
3
4
5 |