| Pages:
1
2
3
..
5 |
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
Sulfuric Acid Production: Revisited
Ways of producing H2SO4 have been discussed many times here on SMDB, but there is still no simple way to produce it.
You have a few choices when trying to get your hands on H2SO4:
1. Buy Super Impure Sulfuric Acid Drain Opener
2. Build the Sulfur Burner proposed by Axehande
3. Electrolyse a Sulfate Salt as discussed by seraniyde.
4. Boiling down battery acid. (DANGER!)FUMES + CORROSIVENESS
There has got to be a better way!
Can this be another possibility...
Sodium Bisulfate seems like an undiscussed route to H2SO4. IT can be found as pH Minus and made from drain opener H2SO4 +NaOH. When added to HCl it
gives off SO2, but that doesn't held much either since you need Vanadium Pentoxide to convert to SO3.
alternatively...WIkipedia says "Sodium bisulfate behaves, to some degree, as if it were a complex of sodium sulfate with sulfuric acid. This is
evident if either the anhydrous form or the monohydrate come in contact with ethanol, which causes them to separate into those two components."
So Sodium Bisulfate with Ethanol will cause a separation into sodium sulfate and H2SO4! BINGO? Sulfuric acid right there no?
The only think I see wrong is that wouldn't that make Ether with the H2SO4 then? If another Alcohol was used would this not happen.
Lastly, check this out...
2NaHSO4 → Na2S2O7 + H2O @ 315°C
Na2S2O7 → Na2SO4 + SO3 @ 460°C
460 Celsius is very achievable.
What do you guys think about the three methods proposed here? I think method two is easiest, but there is the Ether problem.
thanks
Mericad
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
The sodium bisulfate / ethanol route has been discussed here before, although I don't know if a final consensus on it was reached. It has been
attempted here. Sulfuric acid really is difficult to make, thank god it is an industrial chemical otherwise most of us would never see it.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by mericad193724
Ways of producing H2SO4 have been discussed many times here on SMDB, but there is still no simple way to produce it.
You have a few choices when trying to get your hands on H2SO4:
1. Buy Super Impure Sulfuric Acid Drain Opener
(snip)
4. Boiling down battery acid. (DANGER!)FUMES + CORROSIVENESS |
That is what distillation apparatus is for, don't go around boiling acids in open pots in the kitchen.
| Quote: |
(snip)
So Sodium Bisulfate with Ethanol will cause a separation into sodium sulfate and H2SO4! BINGO? Sulfuric acid right there no?
The only think I see wrong is that wouldn't that make Ether with the H2SO4 then? If another Alcohol was used would this not happen.
|
Sorry, it will happen with most alcohols, or the alcohol will dehydrate to a corresponding alkene. I've made di-isopropl ether from isopropanol (kids,
don't do this at home, it forms peroxides as you watch).
I may have to try this. I think the way to go would be to chill everything, filter off the Na2SO4 after allowing the reaction to occur, add a measured
ice water and removed the alcohol under reduced pressure. You'll still end up with somewhat dilute H2SO4, and still need distillation gear. Or can
ethanol be distilled from strong H2SO4 at low pressures without problems?
| Quote: |
Originally posted by BromicAcid
Sulfuric acid really is difficult to make, thank god it is an industrial chemical otherwise most of us would never see it. |
This is too true. Even the old 19th century chemistry books say that making H2SO4 is an industrial process.
One more alternative, although not OTC, would be to use selective ion membranes in a form of electrodialysis. For example, using a solution of sodium
sulfate you would generate sulfuric acid and sodium hydroxide. Both would be contain some Na2SO4, and would not be very concentrated. Running each
solution separtly through the electrodialysis would remove all but the smallest amount of Na2SO4, after which conventional concentration methods would
be needed to get solutions strong than around 30%. This would also work with NaCl to make NaOH and HCl, and ammonium sulfate to make aqueous ammonia
and sulfuric acid.
I've never tried to track down the membranes, so I've no idea how expensive they are; although I know that small units for laboratory purposes are
made. It takes more energy, and expensive electrical energy at that, to make the products that the conventional industrial processes, so it is not
used for that purpose except in special cases.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Boil down battery acid.
I assume you want reasonably concentrated acid. All methods of production "from scracht" will either give you dilute acid that has to be boiled down
anyway or be far more dangerous (*) to work with than boiling down battery acid.
I have boiled down battery acid a few times and the distilate was barely acidic at all, meaning that all the acid remains in the boiling solution. I
think the slight acidity in the distilate was due to the mist formed by boiling, some of which reached the condensor. The only concern here is that
you have hot concentrated acid in the boiling flask, if it breaks or spills...
Sometime ago I posted a concentration table for those boiling down battery acid. I even attached a spreadsheet. It's here:
http://www.sciencemadness.org/talk/viewthread.php?tid=510&am...
(*) Axehandle's (brilliant anyway) method involves the production of SO3 at about 450ºC at some point. If boiling-hot concentrated sulfuric acid has
been called "liquid chainsaw", SO3 at 450ºC could be called "gaseous chainsaw".
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Mericad, there's one more - freezing. Has anyone tried this? I've been meaning to try dry ice which I can get for $1 per pound. I think sulfuric acid
freezes very slowly, though. It also may take a couple of cycles of removing crystals, melting, and re-freezing. The monohydrate (about 86%) has a mp
of near 8 C, and anhydrous is near 10 C, with a pretty flat transition between. It's very easy to boil down to the monohydrate (about 105 C), VERY
hard to remove water beyond that by heat. So that could make for a simple scheme. In principle, it could be done in a regular freezer, but I put about
5 ml of the brown stuff in a -20 degree freezer for 8 hours and nothing..
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
So far as I'm aware threre is not "society for the prevention of cruelty to alcohols" so if they get trashed to ether and or ethene I don't care.
Ethanol is cheap enough.
"460 Celsius is very achievable."
That's probably a moot point, but if you are happy enough with SO3 at 460C then distilling H2SO4 should be a walk in the park.
Dilute sulphuric acid isn't that hard to make; SO2 (from burning sulphur) and aqueous H2O2 would work nicely. SO2 and ozonised air or ordinary air
over a Pt catalyst works fine, but trapping the O3 is a problem.
I guess the easy way is to buy battery acid and boil out the water or distil drain cleaner (I don't think that idea's very apealing unless your "lab"
is separate from your house)
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
I think I will try to boil down Battery Acid. I just bought 900ml for $4 US, its around 40% so that is a good and cheap start. I know boiling it is
pretty nasty because it bumps a lot. I have a borosilicate distillation setup (Graham Condenser, 500ml RBK).
Not_Important: I got real glassware, boiling H2SO4 in the kitchen in a stainless POT is a painful suicide! 
Freezing sounds too easy. I don't have dry ice, where can you get it?
Will hot conc. acid frost up my glassware? Will a Cooking Oil bath suffice or do I need Sand?
Some of you have done this (beerchloride mentioned it once), any tips?
Mericad
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
It will not damage your glassware.
Use boiling stones!
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Ya'll check the good peeps out at www.postapplescientific.com
They'll hook ya up with 98% ACS grade sulfuric for $13.80 per liter if you buy a 4 pack.
And the best part is they ship it ORM-D so no Hazmat Fees!
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
The only boiling stones I got are CaCO3 chips and those obviously won't go well with H2SO4. I heard broken glass works too (break some glass bottles).
What is special about a sand bath? Can you use any other try of powder material that doesn't decomp such as Plaster of Paris or Na2CO3?
I will do distillation of Battery acid tomorrow if I can get this cleared up. (With Pics!)
Mericad
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Even strong H2SO4 will not damage borsilicate glassware.
Sand because it is inert and it is not a powder. Powders tend to cling to the glassware, and often are worse heat conductors than sand (which isn't
that great). Sand is also much less expensive than those alternatives you named.
Inert, unlike plaster of paris which absorbs water from the air, or sodium carbonate that reacts with acids.
Table salt might work, but compare its thermal resistance to SiO2.
Calcium carbonate is generally a lousy boiling stone. Reacts with acids, and is basic so it can futz up some organics. Broken glass is OK, but not as
good as high surface area boiling stones. Not that we can affort it, but once platinum wire was recommended as an anti-bumping device when heating or
distilling sulfuric acid.
Battery acid is reasonably strong, some spa & pool "ph Down" is 10% sulfuric acid.
The boiling point of 80% sulfuric acid is close to 200 C, 40% close to 105 C
Melting Point: 3C (100%), ~ -20 (96%), -32C (93%), -38C (78%), -64C (65%)
Also see
http://www.inorganics.basf.com/p02/CAPortal/en_GB/portal/Anorganische_Basen_/content/Produktgruppen/Anorganische_Basen_(Laugen)/Produktinformationen/S
chwefelsaeure
http://www.colonial-chemical.com/pdfs/sulfmsds2.pdf
Edited to add: While you may have proper glassware, I've read postings elsewhere of people that were going to did try to use glass/ceramic pots on
the stove for boiling down battery acid.
[Edited on 26-10-2006 by not_important]
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The only boiling stones I got are CaCO3 chips and those obviously won't go well with H2SO4. I heard broken glass works too (break some glass bottles).
|
Method for Making Boiling Stones
Clean and efficient boiling stones are easily prepared by melting pieces of glass wool held with forceps in the flame of a Bunsen burner until a
sintered chip with a smooth surface is formed.

[Edited on 26-10-2006 by leu]
Chemistry is our Covalent Bond
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
For good boiling stones the easy and cheap way:
Get an unglazed clay flower pot and break it into small pieces (~ 1/8" dia).
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
WhotDaL...
I did some searching, and didn't find this method, so let us toss Yet Another Route to Sulfuric Acid out.
There's a proposed method of generating hydrogen thermally. H2SO4 is heated about 800 C to produce O2, H2O, and SO2. This is then mixed with iodine
and H2O at ~120 C, the resulting Bunsen reaction leaves the O2 alone and produces HI and H2SO4. The H2SO4 is recycled back into the decomposition
stage. The HI is heated to ~ 350 C to break it down into H2 and I2, with the I2 going back to the Bunsen stage.
So what if we replace the H2SO4 decomposition with a sulphur burner, and the HI decomposition with an air oxidation of HI tp H2O + I2 ?
I must confess I haven't read enough on the Bunsen reaction to see what conditions are eneded to get good yields and allow easy separation of the HI
and H2SO4. Perhaps this could be make into a dual catalyst system I2 serving to transform SO2 and H2O into H2SO4, and (something) + O2 converting HI
into I2 and H2O.
maybe a dumb idea, I need to look at the reaction specifics in more detail.
oops:
http://gcep.stanford.edu/pdfs/hydrogen_workshop/Schultz.pdf
http://npre498.ne.uiuc.edu/NPRE%20498%2005%20Lect%209C.pdf
[Edited on 26-10-2006 by not_important]
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
About boiling stones:
I never cease to be surprised by some good members of this board mentioning broken glass or other nonporous materials as boiling stones. Some mention
the sharp edges as beeing good nucleating points.
In my experience, the result using porous ceramic are so much better than nonpourous materials that, comparing the results, is hard to believe that
any nonporous material is working at all. Porous materials give a flow of bubbles like those aquarium stones.
My oppinion is that boiling stones have to be porous enough to trap some air or keep some (gaseous) vapour inside.
This opinion is backed up by two facts I noticed while doing experiments, both also mentioned in books like Vogel's or Zubrick's:
1- Boiling stones are no good in vacuum distillations. In this case you must use a capilar air inlet. If it was a matter of sharp edges or rough
surfaces, why would it be like that?
2- Boiling stones decrease activity sharply or completely if the boiling solution is cooled and then brought to boil again. You must add new boiling
stones. Again, this does not make sense under the sharp-edges-as-nucleating-points theory.
Also, IIRC, Vogel (third ed.) mentions the use of an iverted closed tube to act as a boiling aid. Like a small test tube (full of air) inserted upside
down in the boiling solution. I never tested this, but is coherent with my oppinion.
My theory about fact 2 is: in the begining of boiling, the porous(air filled) stone gives a liquid/gas inteface where the vapours can expand and
create bubbles. Eventually the air is substituted by the liquid's vapour which still provides an interface for the bubbling. But after cooled, the
vapour condenses and the liquid invades all pores, renderig the boiling stones useless.
I also tried teflon chips, but they floated. Good boiling stones must create their bubbles at the bottom, not only because there is where the hot
spots are, but also to create currents that prevent their formation.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
While a lot of places go along with the idea that it is the trapped gas that is the secret, t the same lab procedure books that say 'it's the gas'
also suggest such devices as sharp platinum bits or powdered glass fused to the inside of the flask, and have references to studies on such 'sharp
point' methods. Go figure.
Some of the sharp edge methods may actually be effective due to gas pockets trapped by the rough surface; the one that had the broken glass be made by
dropping red hot glass scraps into cold water and then oven drying them would certainly seem to lead to a lot of gas filled microcracks in the glass
fragments.
Cheap earthenware pottery may have problems when used with strong acids, although working well with organics. Unglazed porcelain, or at least mostly
unglazed, is better.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There are at least two main ways that boiling stones can work , porosity providing for adsorbed gases or a solvent which can act as a vapor source ,
being driven to evolution at somewhat below the boiling point of the surrounding mixture ......combined with mechanical movement of the stones against
each other caused by
the effervescence , the motion of sharp contact points
of the hard boiling stones causing miniature hot spots
by frictional contact , which results in a microenvironment
where boiling point is exceeded . The motion of the stones as they begin to rattle against each other in
the boiling liquid then further enhances the effect .....
and it does occur in a vacuum also . Fairly coarse granules of bone charcoal which have been presoaked
in toluene or benzene can work well as boiling stones
for vacuum distillations of organic materials . Broken
pieces of terra cotta flower pot , treated the same way
and mixed with the charcoal , and even some fairly large
pieces of broken glass , can together or separately be
effective , at slightly different induction points where
the effect is purely mechanical as for the glass , or
a combination of offgassing and mechanical as for
the porous materials . And then also there is the
reactive sort of boiling stone which may be metal or mineral and is partly consumed in the process , by
reaction with the component being boiled .....if some
sacrificial loss is acceptable for taming the boiling of the
mixture . A darker color and more heat absorptive property
for the boiling stone is also beneficial , as it is desired for the boiling stone to get hotter more quickly than the liquid in which it rests , so
that it acts as a local heat source in contact
with the liquid to be boiled .
[Edited on 26-10-2006 by Rosco Bodine]
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
I just tried my fist attempt at boiling battery acid to concentrate it....I didn't even get it to boil before I had to abort.
I was using a small kitchen pot that has that non-stick coating on the inside and some type of paint on the outside filled with fine sand. On this I
put my 500ml RBF. For boiling stones I broke a bottle and took about 5 1/4in pieces and scratched them up with 220 sand paper. These should provide
plenty of nucleation points. The heat source is a 1000W hotplate that gets RED hot. After about 5 minutes of heating there was copious amount of light
grey smoke coming from the sand or inside of the sand pot, can't tell which one. It smelled terrible so I stopped heating and called it a day.
Do you think it is the non-stick coating decomp. since the sand if not very good heat conductor so the pot got hotter than it is supposed to?
I will try again tommorow if I can firgure out the problem.
Mericad
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Your sand was probably contaminated with some organic matter.
If your hotplate gets red hot then you probably do not need sand bath at all. Use air bath instead - just fix your RBF half inch above hotplate.
For boiling stones i use pieces of broken kitchenware. In my experience glass, even if heavily scrached is not good enough. White clay? (i do not know
how it is actually called in english) of which common mugs and soup plates are made is very good if pieces have enough porous area without that common
enamel coating.
You also need very good ventilation. In my opinion - fume hood or do it outside. SO2 is not fun at all.
[Edited on 26-10-2006 by chromium]
When all think alike, then no one is thinking. - Walter Lippmann
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
All three - teflon type coatings start to outgas at about 220 C, mostly short chain polymers, at around 300 C - distilling temperature for H2SO4 - the
fluorocarbon starts to break down into an interesting and fairly toxic mix.
The colourful outside coating on cookware can outgas at 400 C and above. Used cookware often has a coating of baked on and oxidised fats that smoke
when heated above 300 C
Unwashed sand usually has organics in it, even washed sand has a little.
So, for sand or air baths use uncoated, unpainted containers or first heat them to 500 C with very good ventalation. Don't use non-stick pans unless
you first mechanically remove as much of the non-stick coating as you can by sanding - perferablly all of it; then do the bakeout. Before using sand
for the first time, wash it with water several times, allow it to dry, then heat it to 500 C or higher; using cast iron pans to hold it and a self
cleaning oven to heat them may work, some self cleaning ovens depend on a catalytic coating and don't get as hot as the earlier models.
Don't boil sulfuric acid around anything metallic that you want to keep unless you have a really good draft pulling the fumes away.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Just a question
| Quote: | Originally posted by evil_lurker
Ya'll check the good peeps out at www.postapplescientific.com
They'll hook ya up with 98% ACS grade sulfuric for $13.80 per liter if you buy a 4 pack.
And the best part is they ship it ORM-D so no Hazmat Fees! |
All VERY tentative but:
I might be able to beat that price if I get some in bulk. I know of a source for 18M H2SO4, GAA, 12M HCl, 16M HNO3, anhydrous hydrazine, both 85%
formic and ortho phosphoric acid, along with many dry chemicals (i.e. oxalic acid). DMF, DCM, acetone, Et2O also are available. Problem is, there's a
large deposit on the containers (several hundred dollars per stainless keg of 15 gallons capacity!). Also, ordering bottles to contain specific
things would be a problem. Some can go in HDPE, but some need glass (i.e. HNO3).
Here's my question though: is there enough of a market for this to justify spending several thousand dollars?
Assuming the above doesn't materialize, I've been meaning to get around to this oleum project. I plan to use a sulfur burner that I've designed myself
using a reservoir of liquid sulfur resistance heated to 120*C with an wick made of a high surface area, inert (mullite actually, some of you may know
it as kaowool) material. Pre-dried air (I have a washing bottle I can set up with conc. H2SO4) will be blown in at one point oxidizing the sulfur to
SO2. That will then be brought into the reaction chamber via the venturi effect*(V2O5 on more of that kaowool) which will be hopefully 2 inches in
diameter of stainless 316 flanged swagelock. That will be insulated and heated to sufficient degree to initiate the self-sustaining oxidation. The SO3
will then be lead into concentrated sulfuric acid reacting to produce oleum, H2S2O7.**
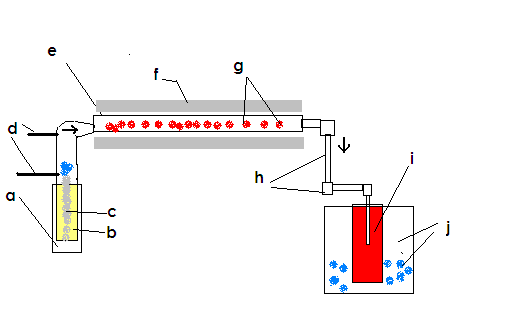
I have hopefully attached a crude pictorial explanation of the intended process.
I have it labeled also, here are what the letters correspond to:
a. heating vessel w/ thermocouple to control temperature
b. liquid sulfur
c. the kaowool ''wick''
d. the dry air inlets from drying column
e. Stainless 316 tube 3 ft/~1m in length by 2 inches/~5cm diameter
f. Insulation to facilitate heating of reaction tube
g. kaowool substrate which has had V2O5 deposited onto it
h. half inch/1.25 cm stainless piping that leads the SO3 into the a bubbler
i. sulfuric acid of approximately 16M concentration
j. cooling vessel filled with ice water
Open to and appreciating any criticism, ideas, and suggestions!
*The air required will be supplied by sending compressed air through sulfuric acid washing bottle. Flow rate to be determined.
**Exotherm of SO3 with water is too great and requires excessive cooling.
[Edited on 26-10-2006 by Fleaker]
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I had not played with molten sulfur for a long time until yesterday when I made some H2S in my hood for making aqueous Na2S. I used
to love to burn sulfur as a teenager in my parents basement. Fortunately I always seemed to oxidize it rather than reduce it! 
Some thoughts:
Does your burner really need a wick? Would a properly sized orifice work just as well or better? In axehandle's thread I made reference to
industrial burners atomizing the molten sulfur, and that the S/O ratio is important for efficient burning.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
not_important, where did you get your mp data? 100% sulfuric acid freezes at +10.4 C.
mericad, not_important is right about the outgassing. Your pan has to be a lot hotter than the temp you want the liquid to boil at. You have to have
"clean" materials. I like the idea of glass pieces for certain things when you want to be sure of purity and non-reactivity. Though contained air
works much better for boiling stones, glass does work (you may have to add 5 or more pieces). I made a simple retort from a glass rod and a broken
vacuum adapter, and found it fairly easy and painless to get to 87% from battery acid, but I noticed that there was a fairly heavy reflux going on in
the flask which did not make it through the retort. I would definitely hesitate to do an open flask boil-down.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
As far as I'm concerned, if it burns lean with more oxygen than necessary, it's of little significance since residual oxygen and any SO3 produced
aren't a bad thing. I'm just hoping that the V2O5 will get the job done. I want to avoid an orifice if at all possible--I'd much rather prefer a
sulfur "candle". Also, how big to make it? What's a feasible (and safe) amount of SO3 to produce? Questions like that still need to be thought
through. I'm looking at the wick part from the stand point of ease and reliability. Since I'm only keeping the sulfur about 10* above its melting
point, it might solidify in the orifice. With a wick, as long as the sulfur is a liquid, capillary action will be on my side.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I don't think a wick type of burner is going to produce enough SO2 output . Something I have considered
is using an old five gallon propane bottle as a combustion chamber . Cut a rectangular hatch door
in the side of it and hinge the cutout as a door piece
after pop riveting some added strips to its perimeter as a flange . Level out the floor inside the chamber with sand or vermiculite , and set in the
middle a ceramic radiant burner element from a gas space heater tilted at a fair incline ~45 degrees with its grid openings upwards , and the open
bottom plugged with about an inch thickness wad of kaowool . The kaowool could be soaked with xylene and ignited to preheat the element to a high
enough temperature so that molten sulfur dripped into the open
grid would inflame immediately and burn away as it flowed downward by gravity across the hot ceramic .
The door could be fitted with a mica or glass window
for observing the combustion and adjusting the sulfur drip until a smooth self sustaining burn rate is accomplished .......almost identically as is
the scheme
used for a waste oil burner .
The ceramic radiants can be gotten from old gas heaters or even ordered from places like eBay , like this one
http://cgi.ebay.com/Tyler-Gas-Heater-Ceramic-Radiant-Grate-N...
or perhaps this one .....I'm not sure which pattern
of element may work best
http://cgi.ebay.com/Gray-Dudley-Gas-Heater-Ceramic-Radiant_W...
And there are embedded electric heating ceramic radiants
which might be better and certainly more controllable
http://cgi.ebay.com/thermoformer-oven-ceramic-element-heater...
Of course you will need a forced air supply and either a blower speed control and /or some sort of valve to regulate the air flow to what is optimum
for the drip rate of the sulfur .
The drip tube would have to be coaxially mounted inside
a much larger and heavier tube and well insulated right up to the tip from where it drips inside the combustion chamber to avoid excessive heating of
the molten sulfur by the combustion chamber heat , which would cause the molten sulfur to thicken and not to drip steadily . A proper sizing
for the molten sulfur feed line and the proper feed rate and temperature would have to be worked out during test runs
to find the optimum settings for everything . Once worked out , the burner should run okay unattended . The catalytic
reaction portion of the reaction could be also worked out so that the the reaction temperature is self-regulating at a particular feed rate from the
combustion chamber . A heat exchanger loop from the catalytic converter could be used
to help keep the sulfur molten in the reservoir with little or
no supplemental electric heating after the system is in operation , the heat energy from the burning and catalytic conversion being sufficient to
maintain the operation of the system . If feed air is being supplied from a compressor maintained supply tank , the air will be sufficiently
dehydrated already , as the water can be drained as
condensate from the bottom of the compressed air tank ,
and the regulated low pressure takeoff air from the high pressure supply tank will already be very dry , having lost its moisture on cooling in the
high pressure storage tank .
[Edited on 27-10-2006 by Rosco Bodine]
|
|
|
| Pages:
1
2
3
..
5 |