| Pages:
1
2
3 |
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Molecular Manipulations  | I'm not a biochemist, but at least I know how to search the web.
Lots of doctors do use silver as an antibiotic coating on medical devices. Many wound dressings containing silver sulfadiazine or silver nanomaterials
may be used on external infections, however there's very little evidence of it's effectiveness. Ref.
Colloidal silver and formulations containing silver salts were used by physicians in the early 20th century, but their use was largely discontinued in
the 1940s following the development of safer and effective modern antibiotics.
There is tentative evidence that silver coatings on endotracheal breathing tubes may reduce the incidence of ventilator-associated pneumonia. Ref.
The silver ion (Ag+) is bioactive and in sufficient concentration readily kills bacteria in vitro. Silver exhibits low toxicity in the human body, and
minimal risk is expected due to clinical exposure by inhalation, ingestion, or dermal application. Ref.
Since the 1990s, colloidal silver has again been marketed as an alternative medicine, often with extensive "cure-all" claims. Again, there's little
evidence that most of these (or any) are effective.
Google is your friend.
As for tin, it isn't very toxic, mostly because in elemental form it doesn't get oxidized and thus remains just tin metal. The compounds of it aren't
as toxic as many other heavy metals, but that doesn't make it "safe" either.
Also I doubt the tin used by Native Americans was free from lead and other actually toxic heavy metals, so their continued use of it may have been
partially from killed brain cells that kept them from realizing they were poising themselves! No offense, it's isn't/wasn't their fault.
[Edited on 16-6-2015 by Molecular Manipulations] |
Off topic, but did you guys hear about the guy that turned blue after ingestion of colloidal silver? He still continues to do it, even after this!
https://www.google.com/search?q=turning+blue+from+silver&...
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Lots of people had that happen to them. I even know of somebody like that, although to a much lesser extent then that. And yeah, despite me telling
her to stop, she drinks the solution on a semi-regular basis.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
There is a world of difference between showing that tin isn't very toxic; and showing that it is actually beneficial.
Incidentally, this "in elemental form it doesn't get oxidized and thus remains just tin metal." is at odds with the fact that tin dissolves in HCl
(which is present in the stomach).
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Is tin actually used by any part of or function in the body?
Is there a list of actual elements that are used by the human body. All I know is that lead is not needed by any living thing.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The process of greying Tin, is aided by the presence of some that has already changed over.
Like prion disease, ice nine, or your more atypical crystallizations .... an errant seed helps the transformation to get started. This seed, then
teaches, or corrupts, the normal Tin.
Process may be hard to start, but once started, it cannot be easily stopped.
On another matter.....Ingest enough Silver, and your skin goes permanently bluish.
"Blue? Like our beloved Lord Krishna?" Some of you will longingly query. Well, No! Not hardly. Stop fantasizing.
The shade of Blue attained, is quite Greyish. Dead body-ish, in fact. If you wish to attain the lovely hue of Lord Krishna, you will have to direct
your quest in some other direction.
[Edited on 16-6-2015 by zed]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Quote: Originally posted by unionised  |
Incidentally, this "in elemental form it doesn't get oxidized and thus remains just tin metal." is at odds with the fact that tin dissolves in HCl
(which is present in the stomach).
|
You're quite right, I was thinking of antimony, which I had just been experimenting with yesterday, and is much more resistant to corrosion by acids.
Sorry about that.
In any case, I never even implied that tin's lack of biological toxicity constitutes my belief that it is beneficial to health. Quite the opposite is
my opinion.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Molecular Manipulations  | Quote: Originally posted by unionised  |
Incidentally, this "in elemental form it doesn't get oxidized and thus remains just tin metal." is at odds with the fact that tin dissolves in HCl
(which is present in the stomach).
|
You're quite right, I was thinking of antimony, which I had just been experimenting with yesterday, and is much more resistant to corrosion by acids.
Sorry about that.
In any case, I never even implied that tin's lack of biological toxicity constitutes my belief that it is beneficial to health. Quite the opposite is
my opinion. |
It's far from clear that Sb is any more inert that Sn
https://en.wikipedia.org/wiki/Antimony_pill
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I fail to see your point.
I specified it's much more resistant to acids, which it is.
Sb can be oxidized by oxygen at high temperatures. So what?
If Sb disolves in the body it must be something besides or combined with HCl (aq).
I recommend injesting neither Sb nor Sn, but to each his own I guess.
[Edited on 17-6-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
"To each his own"
Yum yum. Eat up! So was my suggested procedure on the first page possible?
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Replace sodium with aluminum and tin with nickel and you've copied one step off a Raney nickel production method.
It probably would work, why don't you try it out?
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Huh, thanks for the connection. I might try it if I get the stuff.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Molecular Manipulations  | I fail to see your point.
I specified it's much more resistant to acids, which it is.
Sb can be oxidized by oxygen at high temperatures. So what?
If Sb disolves in the body it must be something besides or combined with HCl (aq).
I recommend injesting neither Sb nor Sn, but to each his own I guess.
[Edited on 17-6-2015 by Molecular Manipulations] |
My point was that the antimony pill works because some of the antimony dissolves "in transit" and acts as a laxative.
So Antimony, even in lumps, doesn't go through the gut unchanged.
The powdered material would be attacked even more so it isn't inert.
Compared with tin, it's likely that less of the Sb would dissolve, but it's a lot more toxic than Sn anyway so it's probably just as bad an idea to
swallow it (if not worse).
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
"Dose makes the poison." An early treatment for syphillis was the arsenical compound Salvarsan.
https://en.wikipedia.org/?title=Arsphenamine
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
And melarsoprol is still used for bad cases of trypanossome diseases.
https://en.wikipedia.org/wiki/Melarsoprol
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
And baseball bats are still used as anesthetic treatments, despite the fact we'd rather not meet one in an alley.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
If the sodium reacts with water you have a hot basic solution and tin will react to sodium stannate, especially in such a finely divided state.
[Edited on 22-6-2015 by Pok]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pok  |
If the sodium reacts with water you have a hot basic solution and tin will react to sodium stannate, especially in such a finely diveded state.
|
That thought has occurred to me too. In the absence of an oxidiser it would be stannite (II), though. Also, although tin is an amphoteric
element, dissolving tin in strong alkali would be a slow boat to China, I think. Other amphoteric elements like Zr and Ti aren't really attacked much
by alkali either.
Of course very finely divided tin would increase reaction speed. For that reason I would not go down that route either.
[Edited on 22-6-2015 by blogfast25]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Pok  |
If the sodium reacts with water you have a hot basic solution and tin will react to sodium stannate, especially in such a finely diveded state.
|
That thought has occurred to me too. In the absence of an oxidiser it would be stannite (II), though. Also, although tin is an amphoteric
element, dissolving tin in strong alkali would be a slow boat to China, I think. Other amphoteric elements like Zr and Ti aren't really attacked much
by alkali either.
Of course very finely divided tin would increase reaction speed. For that reason I would not go down that route either.
[Edited on 22-6-2015 by blogfast25] |
What about a large quantity of rather dilute HCl solution, instead of water? This might work in small quantities.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Considering reducing Sn(II) in solution is easy, I think of the whole idea as a waste of good sodium metal, frankly.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
OK. Would there be any good application of such a method for any metal? Would it work for more inert compounds, or is the use of aluminum better
suited to the job?
|
|
|
Kagutsuchi
Hazard to Self
 
Posts: 51
Registered: 21-6-2015
Location: Hungary
Member Is Offline
Mood: Happy
|
|
Such method for any metal does not exist in fact, because there are ones that are just too positive to be reduced and the only metal that could reduce
them is francium.
By the way, the method with reducing the ions of the metal should work for most metals, just check the standard potential of them.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Are you sure? According to atomistry "the metal dissolves in warm concentrated alkalis with formation of alkali stannate and evolution of hydrogen.
Stannate is formed rather than stannite owing to the superior acidity of stannic tin".
But the next part is interesting: "indeed a solution of stannite decomposes on concentration into stannate and metallic tin."
So maybe it's possible to get very finely divided tin from disproportionation of stannites.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Pok:
Atomistry is crap, frankly speaking. Very short on actual references/sources.
The oxidation of tin to stannite is by water (H<sup>+</sup> to use the electrochemical notation), and that can only oxidise Sn(0) to
Sn(II) (see e.g. oxidation of Sn to Sn(II) by dilute HCl):
Sn(0)(s) + OH<sup>-</sup>(aq) + 2 H<sub>2</sub>O(l) === > Sn(OH)<sub>3</sub><sup>-</sup> (aq) +
H<sub>2</sub>(g)
Of course Sn(II) is quite easily oxidised to Sn(IV) by air oxygen.
As regards the disproportionation of Sn(II) to Sn(0) and Sn(IV), I've prepared stannite solutions in the past and never saw that, for what that's
worth of course.
[Edited on 23-6-2015 by blogfast25]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Which?
My source: https://books.google.de/books?id=LTTyCAAAQBAJ&pg=PA62
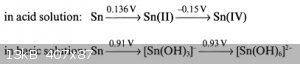
If stannites decompose to tin an stannates in hot alkaline medium, then you will get stannates and not stannites.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
SRPs is thermodynamics and thermodynamics says nothing about kinetics. What is thermodynamically feasible doesn't necessarily happen. I
didn't observe disproportionation of stannite solutions.
|
|
|
| Pages:
1
2
3 |