| Pages:
1
2 |
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fyndium  | | I've found activated carbon a decent anti bump agent. Inert to mostly everything, perhaps excluding HNO3 and some strong oxidizers, heavy enough to
stay at the bottom, as porous as you can get, easy to separate and also otc, cheap and ready to use straight from the bag. |
Silica gel also works but carries a little water into the still.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
99% ethanol is sold as bio-ethanol here to be used in decorative fireplaces. It also only contains MEK and denatonium. You can distill the first part
as 96% and as anhydrous after that.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
In general water solutions containing significant dissolved CO2 do not bump.
A pinch of sodium bicarbonate can be added to a water bath to prevent bumping. That works because the bicarbonate produces dissolved CO2.
[Edited on 8/18/2020 by wg48temp9]
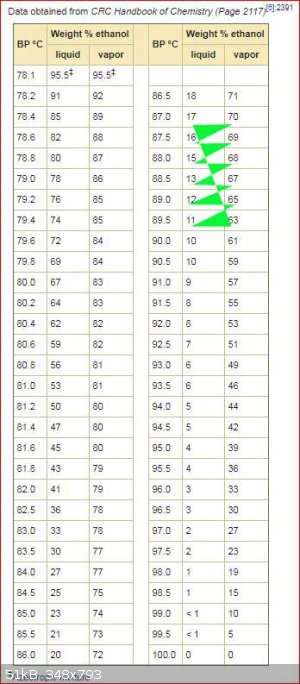
The OP asked how much 40% alcohol can be obtained from 17% alcohol by a simple distillation.
Below is table of boiling points of alcohol water mixtures with the composition of the vapour at that boiling point.
If you look at the entry for 17% alcohol the vapour has 70% alcohol. which reduces the percentage of alcohol in boiling liquid. When the percentage
of alcohol in the boiling liquid reaches 16% the percentage of alcohol in the vapour is reduced to 69%. The zig zag line shows how the percentage
changes as the distillation progresses. You stop the distillation when most of the alcohol has been boiled off or when the temperature gets to 100C.
This relates to a simple distillation with no fractionation column.
PS: sorry I screwed up the zig zag line hopefully you can still follow it with the green triangles
.
[Edited on 8/18/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
| Pages:
1
2 |