alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
Acid in Large jug, how do I use a small amount?
I have a large jug of Sulfuric acid that I've been somewhat hesitant to use. http://www.brew-winemaking.com/productimages/6271.jpg
It's stored in a jug just like that, but I don't know how I would use small amounts. Would I pour a little bit in a smaller jar, then measure it out
using a Graduated Cylinder? I only have my beakers and flasks I use. Unless I could use a small Mason jar to hold a smaller amount, I dunno. I suppose
I should use a funnel too.
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Big syringes like the ones used for injecting marinades into meats come in handy for these sorts of things. Plastic aquarium tubing can be attached
to the end of the syringe, and then the amount needed can be drawn out of the jar with no problem. A lot of the syringes are even calibrated in ml's,
so that makes things even easier.
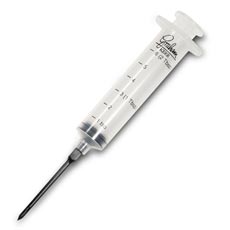
[Edited on 26-1-2007 by Hilski]
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
Ok... Any suggestions as to where I might find such a syringe OTC? Grill and/or meat stores?
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Most all grocery and department stores have them in the kitchen gadget section, with the measuring cups and candy thermometers and such. Sometimes
they also come packaged with bottles of meat marinade.
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
If I could make a suggestion, as all my teacher always told me: always pour a small amount in a small beaker, never enter anything in the mother
solution. You do this to not contaminate the big jug with any impurity that could be present on your tool. When you want to use some, just pour a bit
in a beaker, you can always protect this beaker for later use if you don't really care about purity.
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
like 60% of the adventure of science doing is just walking around with your eyes open looking for stuff
|
|
|
alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Blind Angel
If I could make a suggestion, as all my teacher always told me: always pour a small amount in a small beaker, never enter anything in the mother
solution. You do this to not contaminate the big jug with any impurity that could be present on your tool. When you want to use some, just pour a bit
in a beaker, you can always protect this beaker for later use if you don't really care about purity. |
Valid argument, however I do believe the "Aquarium tubing" attached to the end of the syringe will be used only for the same liquid. Then will be
thrown out.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Even then, I would strongly recommend against using such tools in the main stock of your chemical. A small mistake is easily made, and it would be sad
if that causes contamination of your main stock.
Take a glass bottle with a good plastic cap and pour some of the acid into the bottle (using a clean small plastic funnel, which can be purchased at
hardware stores for pouring motor oil). Then cap your bug jug and use the contents of the bottle, until it is empty. Pouring acid out of the bottle is
easy, you can pour directly into a test tube and use the syringe from that if you want precisely measured volumes.
For all of my chemicals, I have a main stock, and a small container for direct use. The small container is refilled from main stock if it is empty. In
this way, I avoid accidental contamination of main stock, and it has the added benefit that the main stock of chemicals is not opened all over again
(some chems are hygroscopic, air-sensitive, or otherwise easily contaminated).
|
|
|
joeflsts
Hazard to Others
  
Posts: 226
Registered: 14-1-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by alyks
I have a large jug of Sulfuric acid that I've been somewhat hesitant to use. http://www.brew-winemaking.com/productimages/6271.jpg
It's stored in a jug just like that, but I don't know how I would use small amounts. Would I pour a little bit in a smaller jar, then measure it out
using a Graduated Cylinder? I only have my beakers and flasks I use. Unless I could use a small Mason jar to hold a smaller amount, I dunno. I suppose
I should use a funnel too. |
I understand. In fact the larger the bottle the more respect it gets from me! 
I find that finding a smaller boston type bottle (amber) works well and you can handle it much better.
It will also keep your stock pure.
Joe
|
|
|
alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
I don't know where to find a suitable bottle. And the amber bottles are way to small. But I'll keep looking.
|
|
|
joeflsts
Hazard to Others
  
Posts: 226
Registered: 14-1-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by alyks
I don't know where to find a suitable bottle. And the amber bottles are way to small. But I'll keep looking. |
You can get them online. Years ago, and I do mean years ago, I ordered some 1 liter amber bottles through my pharmacist.
Joe
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hilski,
I am becoming impressed with your improvisation! Here, we call those things "Cajun Injectors" and they can be purchased everywhere from Wal-mart to
Ace hardware (where you can also get a nice rig for, er, turkey frying). Make sure to avoid the el-cheapo so that you get a stainless needle.
For H2SO4 just about any bottle will do so long as *there is no water involved*. "Normal" glass will fail if you attempt a dilution (or water
otherwise is introduced) therein. The cap is another matter. Usually, the acid is sold with a bakelite-type cap with a teflon liner (teflon tape will
not do it). PE is probably OK for the short-term, just dispense only what you think you will need for a few days. A standard PE plastic funnel will do
for transfer.
Wear gloves, glasses, and maybe a raincoat in case of catastrophic spillage. If you spill it on yourself, *do not* immediately hit the shower, remove
your clothes (and the bulk of the acid which becomes boiling hot on contact with water) as quickly as possible--then hit the shower.
In fact, now that I remember, I have seen H2SO4 (conc.) actually stored in PE (maybe PP) bottles when transferred from drums (industrial grade).
Take care,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I also have a smaller container for every chemical whose bottle is too big for everyday lab use.
For the H2SO4 it is a 250ml thick-walled PE bottle in which I once ordered 250ml of H2SO4 (in my early days when 250ml would last me for half a year).
For specially purified solvents like absolute Ether it is a 100ml brown glass bottle from the pharmacy, with added aluminium foil lining in the cap
against vapor diffusion.
I only dry 100ml of ether at a time (predrying with CaCl2, then filtering and intensive drying with Na/K followed by distillation), because the dried
ether is unstabilized (but it is stored over finely divided Na/K-alloy, which should protect from peroxides somewhat) and I only do small-scale
grignard reactions.
|
|
|
alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
Ok, I found some Amber bottles at a Herb shop. They have a plastic cap with a little clear plastic looking thing in the cap that says "Polyseal". A
google revealed it to be PE. I'm assuming that the cap, with or without the Polyseal, will be suitable.
|
|
|
Waffles
Hazard to Others
  
Posts: 196
Registered: 1-10-2006
Member Is Offline
Mood: No Mood
|
|
Use a frickin turkey baster. I don't know why contamination is such a big issue- just wash it and rinse with distilled water.
\"…\'tis man\'s perdition to be safe, when for the truth he ought to die.\"
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
| Quote: | Originally posted by garage chemist
I also have a smaller container for every chemical whose bottle is too big for everyday lab use.
|
This is EXACTLY what I do. I have a little "chemistry set" like when I was a boy. Every reagent, element, etc is represented in a small container and
set aside for use. The big bottles are safely stored respective of their content (caustics, famables, high toxics, etc) safely away from both kids,
food, pets, theft.
It's embarresing to admit but aside from goggles, gloves, and temperary containers the one item I learned to use the hard way was a lab apron or lab
coat. I once got very sick by unknowingly wiping gloved hands on my pants and contaminating my bare hands again much later & consuming food. A
very small amount of some materials can put you in the hospital or worse.
[Edited on 28-1-2007 by quicksilver]
|
|
|