| Pages:
1
2 |
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Extracting chromates from stainless steel
So i've been following the process in this Extractions and Ire video https://www.youtube.com/watch?v=F_W-IyUTM5M
I've dissolved stainless steel cutlery with hydrochloric acid over the period of a week or so using a heat plate and a bucket and adding water and
acid as needed.
Eventually I removed excess metal and then netralised the acid and dropped out iron hydroxide and chromium carbonate by adding sodium carbonate to
the bucket.
I then reacted all of this with calcium hypochlorite to leave me with calcium chromate in solution and hopefully everything else insoluble.
I filtered and got a nice clear yellow solution which I presume was calcium chromate.
I boiled some of this to dryness and did get some yellow solid but there was a dark green precipitate that also dropped out. I've since redissolved
everything but I no longer had a nice yellow solution but a dark cloudy solution.
I've now filtered this and have a clear yellow solution again but not as bright yellow as it was.
Anyone have any idea what this dark green material is?
|
|
|
bnull
Hazard to Others
  
Posts: 475
Registered: 15-1-2024
Location: South of the border, wherever the border is.
Member Is Offline
Mood: Fighting the banking system and international commerce. Still losing.
|
|
Someone else is having similar problems with chromates here, if you're interested.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Yeh it could be chromium chloride hexahydrate.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Oops! sorry, I deleted the post to fix it then more posts appeared.. SORRY
Chromium(III)chloride octahydrate, dehydrated to green hexahydrate?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Urgh I think I have a very impure solution. I keep boiling this down and it's yellow but now I keep getting white precipitate coming out.
I might give up on this.
|
|
|
fx-991ex
Hazard to Self
 
Posts: 99
Registered: 20-5-2023
Member Is Offline
|
|
Convert it to the potassium dichromate, it has a very low solubility.
I guess once purified you can convert it back to the chromate by adjusting ph.
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Beautiful thing about most inorganic chemistry is you can burn it then start from the beginning. Got some scraps im going to dissolve here soon.
"You can't do that" - challenge accepted
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Ok so eventually the yellow solution goes kind of oily looking and then starts to dry, i've stopped heating and now have some yellow solid which i'll
try and dry now.
Just not sure about the colour, I can hit that with a gas torch and it looses water and then remains yellow.
[Edited on 24-3-2024 by Chemgineer]

|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
I'm going to have another go starting from chrome powder to simplify things a bit.
The above doesn't change colour with addition of hcl.
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Quote: Originally posted by Chemgineer  | Eventually I removed excess metal and then netralised the acid and dropped out iron hydroxide and chromium carbonate by adding sodium carbonate to
the bucket.
I then reacted all of this with calcium hypochlorite to leave me with calcium chromate in solution and hopefully everything else insoluble.
|
Did you skip a step?
The carbonates should next be brought back into solution at a ph of 1 and filtered again to remove the iron, then the ph raised to 7 before the
hypochlorite addition or im thinking if something else?
"You can't do that" - challenge accepted
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
The remaining undissolved steel I just removed in large pieces. I'm not sure what lowering the ph would achieve?
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
The solubility of chromium carbonate increases in an excess of NaOH. Allowing it to be seperated from the iron catons
"You can't do that" - challenge accepted
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Rainwater  |
The carbonates should next be brought back into solution at a ph of 1 and filtered again to remove the iron, then the ph raised to 7 before the
hypochlorite addition or im thinking if something else? |
Quote: Originally posted by Rainwater  | | The solubility of chromium carbonate increases in an excess of NaOH. Allowing it to be seperated from the iron catons |
Adding NaOH would be raising the pH. Probably to 13-14. Then it would need to be lowered to ~7 after filtering, by addition of acid. Not sure
if you're confused or just making typos.
|
|
|
Admagistr
Hazard to Others
  
Posts: 372
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Rainwater  | | The solubility of chromium carbonate increases in an excess of NaOH. Allowing it to be seperated from the iron catons |
But chromium carbonate cannot be isolated from aqueous solution, because it undergoes rapid and complete hydrolysis to chromium hydroxide, possibly
CrOOH and carbon dioxide! In the same way, aluminium carbonate cannot be precipitated! I have tried this many times and it always releases a lot of
CO2, even the literature says that chromium carbonate cannot be produced this way...
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
If you lower the pH everything goes back into solution. Raising the pH the chromium will go back into solution and the other metals will remain as
solids. It does not matter if the chromium is in the form of carbonate or hydroxide, both increase in solubility as the pH increases.
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Quote: Originally posted by Admagistr  | Quote: Originally posted by Rainwater  | | The solubility of chromium carbonate increases in an excess of NaOH. Allowing it to be seperated from the iron catons |
But chromium carbonate cannot be isolated from aqueous solution, because it undergoes rapid and complete hydrolysis to chromium hydroxide, possibly
CrOOH and carbon dioxide! In the same way, aluminium carbonate cannot be precipitated! I have tried this many times and it always releases a lot of
CO2, even the literature says that chromium carbonate cannot be produced this way... |
Thanks! I've currently got some on my vacuum filter.... i'll stop that!
|
|
|
Admagistr
Hazard to Others
  
Posts: 372
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Thanks! I've currently got some on my vacuum filter.... i'll stop that![/rquote]
Freshly precipitated Cr(OH)3 can also be dissolved in alkaline solutions! Like KOH, NaOH. Just when calculating and weighing the substance do not
consider chromium carbonate, but Cr(OH)3, it is different and the ratios of weighed substances would be different. So you don't have to pause the
project!
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
little of both is normal for me. I got the ph scale
upside down and half backyards again.
So going way back to the beginning, im suggesting that the iron contamination may be an issue. To remove the iron
1) with a lot of stainless steel disolived in some hcl acid. The solution would then be filtered to remove the carbon and molybdenum that dont want to
disolve
2) addition of saturated sodium carbonate solution should produce a nice percipitate containing the chromium, iron, nickel.
Filter.
3) take the ... cant remember the word.... oppsite of filtrate... solids and disolve them in sodium hydroxide solution, this should produce a nice
percipitate of iron and nickel oxides or hydroxides or who knows what but you can filter it off.
Filter again.
Then procede with neturalizeing the solution and adding the bleach,
Sorry about the poorly written and confusing responce from earlier
"You can't do that" - challenge accepted
|
|
|
bnull
Hazard to Others
  
Posts: 475
Registered: 15-1-2024
Location: South of the border, wherever the border is.
Member Is Offline
Mood: Fighting the banking system and international commerce. Still losing.
|
|
Oversize. I always thought that the filtrate was the solid stuff (ah, yes, and poor me used it in the forum with that sense). Call it "the
solids" and you're safe.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Residue. Filter cake.
There is probably a more technical word, but that is what I use.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 226
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Precipitate is the word. (in analytical chem, after filtering you got the filtrate and the precipitate Or at least is so written in books)
Im too fighting with chromates. What I learn so far:
Hydroxides require ph control. starting ph4 hydroxides start to precipitate. as ph got to 6 they change color from green to a more green blue in
water. and iron starts to oxidize by air ( Fe2+ to Fe3+) and you will start se orange rust color.. If you go too alkaline because of using NaOH
excess, amphoteric metals (in this case Chromium will redisolve). the best is to keep ph below 9. I think this is why carbonate are used, you cannot
go beyond ph 7+ , but you will add a lot of it. (this is problem for future on reducing by boiling, as a lot of salt will be precipitate)
Now Im in the oxidizing step. Once I get it right will post results.
Go SAFE, because stupidity and bad Luck exist.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 226
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Found this very good read to understand Hydroxide ph precipitation.
https://nvlpubs.nist.gov/nistpubs/jres/30/jresv30n2p89_A1b.p...
ANALYTICAL SEPARATIONS BY MEANS OF CONTROLLED HYDROLYTIC PRECIPITATION By Raleigh Gilchrist
(although costly indicators are used, today with cheap Ph meter, could be "easily" done)
Nickel.-Bivalent nickel is quantitatively precipitated as a palegreen gelatinous hydroxide at the end point of cresol red. It is
likewise insoluble at the end point of thymol blue.
Cresol red (pH indicator) below pH 7.2 above pH 8.8
Iron. - Precipitation of hydrated ferric oxide is quantitative from
the end point of brom phenol blue
Bromophenol blue (pH indicator) below pH 3.5 above pH 4.6
Chromium.- Characteristic tervalent chromium hydroxide is quantitatively precipitated at the end point of thymol blue
Thymol blue (pH indicator) below pH 8.0 above pH 9.6
Also from another source find attached graphic
https://heienv.com/hydroxide-precipitation-of-metals/
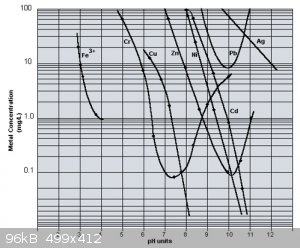
Go SAFE, because stupidity and bad Luck exist.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RU_KLO  |
If you go too alkaline because of using NaOH excess, amphoteric metals (in this case Chromium will redisolve). the best is to keep ph below 9. I
think this is why carbonate are used, you cannot go beyond ph 7+ , but you will add a lot of it. (this is problem for future on reducing by boiling,
as a lot of salt will be precipitate)
Now Im in the oxidizing step. Once I get it right will post results.
|
Carbonate solutions can go beyond pH 11.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 226
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
Yes, but in this case, my experience with carbonate and more with bicarbonate neutralizing this chloride/hydroxide solution is that ph does not go
further than 7 (because you use CO2 bubbling as end point) and once you start reducing by boiling you get lot of salt precipitation.
Go SAFE, because stupidity and bad Luck exist.
|
|
|
Chemgineer
Hazard to Others
  
Posts: 216
Registered: 25-5-2021
Member Is Offline
|
|
Quote: Originally posted by Rainwater  | little of both is normal for me. I got the ph scale
upside down and half backyards again.
So going way back to the beginning, im suggesting that the iron contamination may be an issue. To remove the iron
1) with a lot of stainless steel disolived in some hcl acid. The solution would then be filtered to remove the carbon and molybdenum that dont want to
disolve
2) addition of saturated sodium carbonate solution should produce a nice percipitate containing the chromium, iron, nickel.
Filter.
3) take the ... cant remember the word.... oppsite of filtrate... solids and disolve them in sodium hydroxide solution, this should produce a nice
percipitate of iron and nickel oxides or hydroxides or who knows what but you can filter it off.
Filter again.
Then procede with neturalizeing the solution and adding the bleach,
Sorry about the poorly written and confusing responce from earlier |
So if i'm now starting with chromium powder and i've neutralised with sodium carbonate and washed the blue/grey chromium carbonate (hydroxide?). I
should be fine to just add a solution of calcium hypochlorite?
Or does it still require some hydroxide to progress?
|
|
|
| Pages:
1
2 |