MountainMan
Harmless

Posts: 20
Registered: 6-4-2011
Member Is Offline
Mood: No Mood
|
|
Sodium Percarbonate H2O2 Titration
I'm trying to determine the amount of H2O2 released from a sample of Sodium Percarbonate using a method in the Journal of Chemical Education (Wada and
Koga 2013- attached). The instructor and student notes use a notation that I'm not familiar with (I'm 75 and took my last chemistry course in the
mid-1960s). Can someone explain the equation in the image of the equation that I attached ? The particular issue is the use of the colon ( symbol since I'm not familiar with it. symbol since I'm not familiar with it.
From the text of the supplement:
"Redox titration of H2O2 (iodometry)
Assuming that the composition of SPC is xNa2CO3∙yH2O2, the stoichiometry of the reactions can be written as
yH2O2 + 2yKI + yH2SO4 → yK2SO4 + 2yH2O + yI2
yI2 + 2yNa2S2O3 → 2yNaI + yNa2S4O6
The volume, V (mL), of the Na2S2O3 standard solution used for the titration can be related to the coefficients x and y in the hypothetical composition
of SPC according to
(see image of equation)
where m0 and c are the mass (g) of SPC in the solution to be titrated and the molar concentration (mol L1) of the standard solution, respectively.
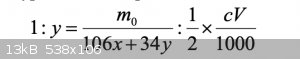
Attachment: Wada and Koga 2013 SodiumPercarbonatePrepAnalysis Suppl 2.pdf (580kB)
This file has been downloaded 140 times
|
|
|
bnull
Hazard to Others
  
Posts: 435
Registered: 15-1-2024
Location: South of the border, wherever the border is.
Member Is Offline
Mood: Dazed and confused.
|
|
It's a proportion. I don't see it around much these days. It translates to
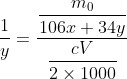
Expanding the equation gives
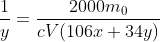
You solve it for y and divide by x to find the ratio of peroxide to carbonate
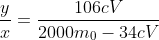
As to why they didn't use fractions, I have no idea.
[Edited on 29-1-2024 by bnull]
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|