B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
preparation of barbituric acid (question about the mechanism)
Hey there, so here's the preparation:
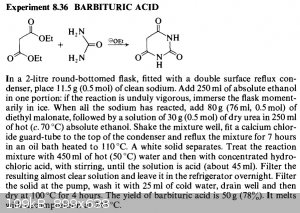
I don't understand the purpose of the NaOEt.
I considered:
1. NaOEt being a catalyst for the reaction (e.g. proton carrier). but won't the urea be enough for this purpose? it's weakly basic after all (even
water are often used as a proton carrier).
2. NaOEt is needed for neutralizing the product (otherwise the urea would form hydrogen bonds with the protons preventing it from reacting). but
couldn't we just use excess urea instead? why go through the troubles of making NaOEt?
I also thought about a few downsides of strongly basic environment:
1. the diethylmalonate getting deprotonated thus becoming less reactive towards nucleophilic attack.
2. water contamination are more likely to screw things up react with the diethylmalonate.
3. decomposition of the urea into ammonia.
to focus on my questions:
1. what is the purpose of the strong base?
2. would the reaction work without it?
3. would the reaction work with excess of urea instead?
much thanks ahead,
B.D.E.
[Edited on 31-8-2021 by B.D.E]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
1. Neutralize the product and catalyze the reaction
2. unlikely, possibly to some degree with a hydroxide base.
3. very unlikely
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Deprotonation is one of the steps in this mechanism of amide formation, and urea is not a strong enough base to do that.
https://www.chemistrysteps.com/esters-reaction-with-amines-t...
[Edited on 2-9-2021 by Tsjerk]
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Well I got a doubt about the synthesis itself. As per the literature they say you will have to add hot water and acidify the precipitate and in the
end you get a clear solution. I did that and I did get a clear acidic solution. Then it says to keep the contents in refrigerator overnight and next
day you'll end up with the barbituric acid solid. This step didn't work out for me and I didn't get any solid.
Is there any logical explanation for that? What could be done to retrieve the barbituric acid
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|