| Pages:
1
2
3
4 |
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Karlos, there is a more straightforward synthesis, utilizing the Azide.
Ortho-Vanillin would be the starting point. Azides are a concern, but folks are doing it.
I've got a reference. I'll go dig for it.......
https://pdfs.semanticscholar.org/ed76/3bf008c6e9ef09537f9fc8...
Page 21, Hemetzberger
As for the synthesis of Indoles via their 2-carboxylates, being decarboxylated.... The method is fairly well known.
I'm going to try to link you, to something relevant ...... https://www.google.com/search?client=safari&rls=en&q...
Click on the science madness link. I can't seem to link you directly. It is a PDF. PDFs drive me crazy. I can't seem to link to them.
As I may have stated elsewhere, the Phenylhydrazone of Acetaldehyde does not yield Indole under ordinary Fischer conditions, but when the
Phenylhydrazone is passed through a tube furnace, or a flow type reactor it may. I assume the 5-methoxy-Indole might be constructed by the
Fischer..... But that is not certain.
Of note. In that general paper on Indoles, there is mentioned a method of reducing Diazonium Salts to Phenylhydrazines, via Ascorbic acid. Looks
like a useful technique. Ascorbic Acid, I can get by the Kilo, at minimal cost. Page 9, Scheme 7.
Diazonium Salts, form useful phenylhydrazones directly, when reacted with Dihydro-Furan. Upon cyclization, Tryptophols are produced.
Those Tryptophols, may be converted to Tryptamines, but the roads are not very familiar to me. And, in years past, the requisite reagents were fairly
arcane.
[Edited on 24-12-2020 by zed]
[Edited on 24-12-2020 by zed]
[Edited on 24-12-2020 by zed]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
A link to the Hemetzberger Synthesis. Just open the PDF.
This synthesis appears to be an effective path to the Indole you wish to produce.
https://www.researchgate.net/publication/305775529_Hemetsber...
[Edited on 31-12-2020 by zed]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
A little off topic but this about synthetic toad venom by Vice on Hamilton's pharmacopoeia (5, Meo-DMT synthysis 30 minutes in) seems ok interesting
https://youtu.be/fFXGa-Ruz_M
[Edited on 31-12-2020 by symboom]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
symboom,
Thanks for the link. Very interesting stuff on Hamilton's. Also, it led me to a Hamilton link, on the story of a Clandestine Chemist.
The gentleman in question, turned out to be an old collegue. Haven't seen young Anakin in decades. I myself, took another path. Wary of extremes,
I suppose.
https://www.youtube.com/watch?v=3cvcoVPYsBE
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I ditched the chance of an interview with hamilton.
Screw vice.
They should at least pay well for such a chance!
Melgar thought different, apparently(they choose him instead then after I refused).
I gave an interview for a dutch-language book about MDMA instead.
The journalist was very nice and knowledgeable and it was fun to answer his questions.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I don't think such fame is good. Once upon a time, it might have caused beautiful young women, to jump in the sack with you. Now-a-days.... Not so
much. Just attracting trouble.
Once again: https://www.researchgate.net/publication/305775529_Hemetsber...
At the moment, you can either read of download the PDF, for free. Just click.
[Edited on 2-1-2021 by zed]
[Edited on 2-1-2021 by zed]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Ortho-vanillin(or its 2-acetylated analogue) and methyl 2-azidoacetate to give the substituted a-azidocinnamic acid, which cyclises in boiling xylene
to 2-carboxymethyl-4-hydroxy/acetoxy-5-methoxyindole.
Yes, that is doable and cheap indeed!
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
What's your plan for this methyl 2 azidoacetate? I forgot to tell you I had bought some ortho vanillin for when a pathway is figured out for this
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
https://prepchem.com/synthesis-methyl-2-azidoacetate/
Methyl chloroacetate or bromoacetate are cheap or easily made.
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
They sure are cheap but I cant find a supplier! The patents are mostly gibberish but from what I could tell it seems like they're reacting methanol
with chloroacetic acid somehow in the vapor phase
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by zed  | | I don't think such fame is good. Once upon a time, it might have caused beautiful young women, to jump in the sack with you.
|
Until you get out of bed to spend three minutes checking on your reaction. ROFLMAO.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
https://scihub.wikicn.top/10.1081/lft-200043689
This should blow away any doubts. Ferric chloride with 90% yield!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by arkoma  | Quote: Originally posted by zed  | | I don't think such fame is good. Once upon a time, it might have caused beautiful young women, to jump in the sack with you.
|
Until you get out of bed to spend three minutes checking on your reaction. ROFLMAO. |
Damn I should have never mentioned that event 
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Reagents often being ardous to obtain, I am curious as to if requisite Chloro or Bromo, acids or esters might be synthesized from amino acids
(or their esters)... via Diazotization.
Glycine is available, and dirt cheap. But perhaps I am being obtuse.
Here is an example, of what I have in mind. http://www.orgsyn.org/demo.aspx?prep=CV8P0119
OK.... I went out scouting..... Now I'm back.
Nope. Probably not gonna work. It seems the guys have tried this out before, to no avail. http://www.sciencemadness.org/talk/viewthread.php?tid=65553
[Edited on 4-1-2021 by zed]
[Edited on 4-1-2021 by zed]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
OK, I thought about it. Some of the guys have reported failure to produce Chloro-Acetic acid via Glycine Diazotization.
Since the desired intermediate, is the Ester of Chloro-Acetic Acid; might it be possible to to produce that Ester,
Via the diazotization of Glycine's Ethyl Ester Hydrochloride?
Well, maybe. I'm gonna look it up. Might not work. Might be a hydrolysis problem. Might be a solubility problem.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Just Wanted to add this video of 5-MeO DMT by Hamilton's Pharmacopoeia.
https://youtu.be/qipKKBmY_LQ
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I am still thinking about a suitable protection group for the ortho-vanillin, to react with ethyl azidoacetate.
I've seen an example at the hyperlab, using benzyloxy-protected salicylaldehyde, for the preparation of 4-BnO indole, but I wonder if a tosyl group
could survive the conditions as well?
Because I can very well replace the tosylate with an acetate later on, with no need for a hydrolysis in between.
And an acetate is something that would likely not survive the hemetsberger conditions, I fear.
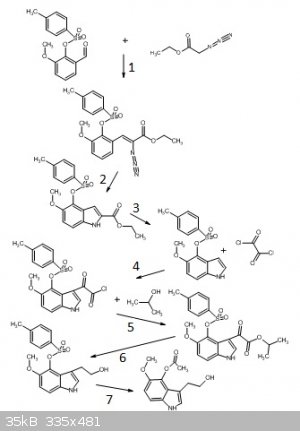
E: I attached a picture of the reaction scheme(sponsored by chemsketch).
The numbering for the reactions: 1 for the reaction which forms the substituted a-azidocinnamic ester, 2 for the thermal rearrangement into the
indole-2-carboxylate ester, 3 for the decarboxylation(actually done in two separate reactions), 4 for the reaction of the tosylated indole with oxalyl
chloride, 5 for the ester formation of the glyoxyl chloride, 6 for the reduction of the glyoxylate ester to the tryptophol, and 7 for the substitution
of the tosylate with acetoxy.
From there on, just a tosylation and substitution.
[Edited on 11-6-2021 by karlos³]
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Do you have the link for that thread?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Search it out for yourself, its [post=556542].
E: here it is https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
[Edited on 11-6-2021 by karlos³]
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Oh I have that thread bookmarked, still have to try that synthesis out. I didn't read through the whole thing though so I must've missed the good
stuff
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Hi, your post inspired me to do a writeup. Link:
https://old.reddit.com/r/TheeHive/comments/nyq8hs/a_relative...
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
But that post is absolute nonsense 
It is also not a writeup(that would be something you've done) but just a synthesis scheme.
And it doesn't work like this.
Have you even read the hyperlab link, or read about the Hemetsberger at all?
To think you can just introduce the functional groups like this on such a sensitive molecule, thats just not realistic at all.
[Edited on 13-6-2021 by karlos³]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Needs references
Reflux condenser?? I barely know her!
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Quote: Originally posted by karlos³  |
It is also not a writeup(that would be something you've done) but just a synthesis scheme.
And it doesn't work like this.
Have you even read the hyperlab link, or read about the Hemetsberger at all?
To think you can just introduce the functional groups like this on such a sensitive molecule, thats just not realistic at all. |
Ah, I'm not a native speaker.
It's not that sensitive, and Hemetsberger rearrangement requires strong heat anyway.
These reactions aren't that unfamiliar to someone knowing organic chemistry:
Fries rearrangement
Mannich condensation
Darzens reaction
Hemetsberger reaction and decarboxylation
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I would love to see a reference for your imaginative tryptamine synthesis via hemetsberger.
Because that is something I am definitely not familiar with.
Its not that sensitive?
Just read the hyperlab report.
|
|
|
| Pages:
1
2
3
4 |