| Pages:
1
2
3
4 |
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
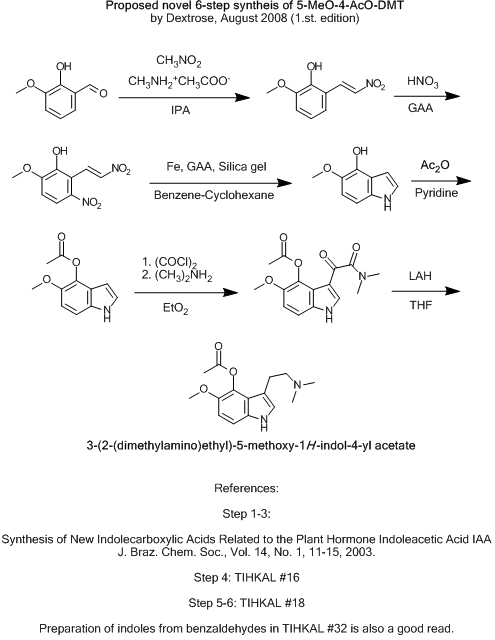
Proposed novel 6-step syntheis of 5-MeO-4-AcO-DMT
I hope this topic will be taken more seriously.
I asked Shulgin about this compound 3 years ago, and he replied the compound indeed was interesting, but the synthesis was to tedious and only one
publication of a 11-step synthesis.
http://www.cognitiveliberty.org/shulgin/blg/index.html
However, after i found this Brazilian publication, i came up with this route.
Synthesis of New Indolecarboxylic Acids Related to the Plant Hormone Indoleacetic Acid IAA
J. Braz. Chem. Soc., Vol. 14, No. 1, 11-15, 2003.
Is available for free here: http://jbcs.sbq.org.br/jbcs/2003/v14_n1/02.pdf
Questions:
Would it be better to nitrate the aldehyde before condensating?
Shulgin does this in TIHKAL #32 when making 5,6-methylenedioxyindole.
HNO3/GAA should yield mostly the ortho-product as far as i have read, compared to HNO3/Ac2O.
Would it be a good idea to use H2SO4 as catalyst when nitrating?
Will the activating groups on ortho-vanillin be any problem, when preparing the indole?
An alternative could maybe be using the O-benzyl-ether of ortho-vanillin as protection group, and making the acetoxy-ester as the last step, like
described here: http://psychedelichosting.info/Ionium/Rhodium/chemistry/psil... ?
The last steps from the indole to the amine, is straight from TIHKAL.
What do you think?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I think you may end up with the 1-hydroxy-2-methoxy-5-nitro-nitrostyrene as the phenol is the most powerful directing group and will result in the
formation of the wrong isomer at the nitration stage.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I think you should read upon electrophilic aromatic substitution and the rules governing its regioselectivity.
The nitration of 2-hydroxy-3-methoxybenzaldehyde gives the 2-hydroxy-3-methoxy-5-nitrobenzaldehyde as the main product (for example, see Bulletin
de la Societe Chimique de France (1975) 2297-2306).
2-Hydroxy-3-methoxy-6-nitrobenzaldehyde has been synthesized by nitrating 2,3-dimethoxybenzaldehyde followed by -CHO group assisted selective
demethylation with BBr3 (see Tetrahedron, 54 (1998) 10007-10016). It can also be synthesized from 2-hydroxy-3-methoxybenzaldehyde if using
O-Ts as protection group (due to the inductive effect of the TsO group the nitration occurs at the para position in relation to the MeO group, see EP176333).
Also, the reduction of the oxamide with LiAlH4 would cause deacetylation.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Bull. Soc. Chim. France (1965), (5), 1411-17 and 1417-1423 also are worth checking as it is the first synthesis of 4-OH-5-MeO-DMT that appeared in
the litterature. Julia did protect the phenol with a benzene sulfonate I remember...
There are also alot other tryptamines in the paper
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Thank you for your replies, guys!
Ullmann, i haven't been able to find that paper, even the university library has problems ordering it home!
Maybe you can help me with that?
I will post an alternative approach, when i've studied your replies some more.
Thanks for the suggestion about the aldehyde, Nicodem.
I forgot to notice that Shulgin's reduction of 4-acetoxyindol-3-yl-N,N-dialkylglyoxylamides caused deacetylation!
I did take note of phenol being very dominat.
Do you think this maybe would be possible?
The idea about possible nitrating in GAA instead of Ac2O i had from this post: http://psychedelichosting.info/Ionium/Rhodium/chemistry/4-ni...
Where there are reported good yields of nitration on the 6-position in GAA.
But yes, it is another aldehyde.
[Edited on 2-8-2008 by Dextrose]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
It seems LiAlH4 doesn't generally cause debenzylation, so that should be possible.
A note on the phenolic nitrostyrene formation, it's possible that this reaction might take more time that with non-phenolic benzaldehydes. Thsi is the
case with the corresponding aldol reactions, and I suppose by analogy the reaction times must be extended here too..
Also, the amine from the indole group might need protection during acetylation, you would need to use a hydrochloride salt of similar?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
One positive thing about this scheme: the starting aldehyde is commercially available & inexpensive. It is called isovanillin and is the
coproduct formed in the manufacture of vanillin from guaiacol.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ritter
One positive thing about this scheme: the starting aldehyde is commercially available & inexpensive. It is called isovanillin and is the
coproduct formed in the manufacture of vanillin from guaiacol. |
No, isovanillin is the trivial name for 3-hydroxy-4-methoxybenzaldehyde. The trivial name Dextrose used ( ortho-vanillin) is actually correct and not just something out of his fantasy. Nevertheless, to avoid confusion it is always better to avoid
nontrivial-trivial-names like this.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
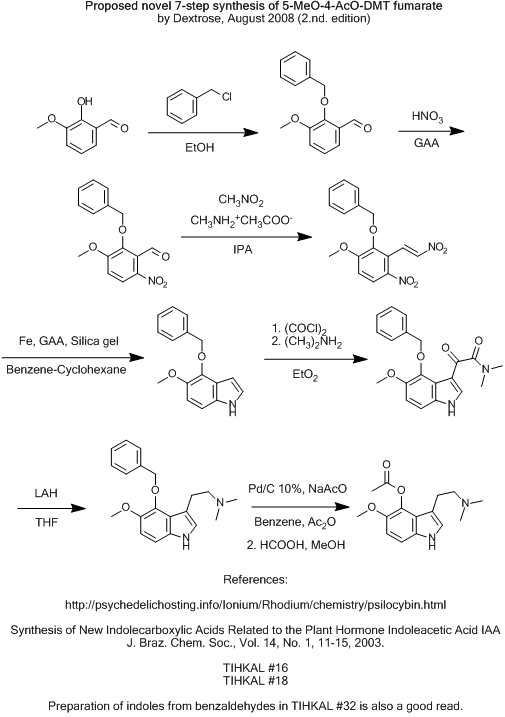
Even though i'm pretty doubtfull myself, i'll post it anyway for flaming purporses. 
Would the benzyl-ether be lesser para-directing, and hopefully place the nitro group on the right position, and not be nitrated on it's own phenyl?
And the big question... would it survive all the way to the final reduction?
Cheers!
I have a third suggestion i'll sketch up soon.
[Edited on 6-8-2008 by Dextrose]
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
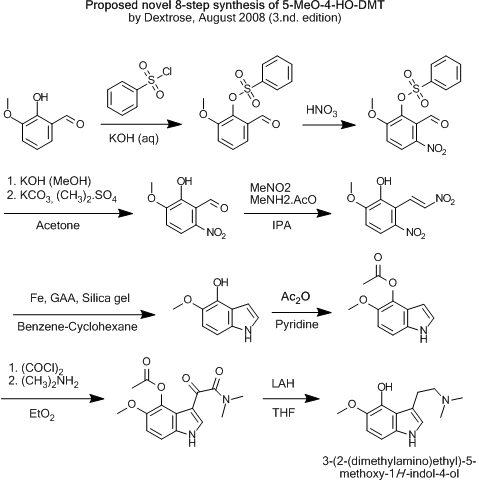
Here's the one i find most likely to work.
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
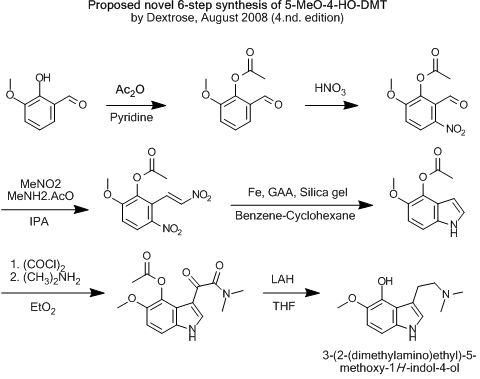
...But if ethers or esters would direct the nitro-group correctly, like a sulfoxide, this would of course be the route of choice.
That would be if the pyrrol-formation is possible in any of the latter two...
[Edited on 7-8-2008 by Dextrose]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I would rather go through the sulfonate, I'm pretty sure the benzyl ether will get removed by LAH, and nitrated by HNO3 (you are nitrating a aldheyde,
which is more desactivated that the benzyl ether..). You could just aswell leave the sulfonate on until the LAH reduction, no need of removing it
straight away and thne acylating. I think LAH can cause complet removal of the sulfonate to the hydrocarbon, so you would need to remove it between
the reaction with oxalyl chloride and the LAH...
What's the purpose of the dimethylsulfate during the desulfonation? That would directly alkyalte your phenol..... A typo maybe?
A little detail, i would perform the initial sulfonation with Et3N or pyridine in EDCM, not aq. KOH as you are dealing with an aldehyde. It would caus
eonly minor side recation, at 0-5°C but it can always be better to have homogeneous mixtures with delicate substrates.
I'm not familiar with the indole ring, but doesn't the secondary nitrogen be prone to some acylation? Or is it unreactive du to the cyclic
conjugation?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Yeah, i must admit that i also find the 3.rd the most plausible.
But 8 steps now, and it would be nice to shorten it down to 7 as you suggest.
Guess it just depends wether it's possible, and which yield you will get, when closing the ring to the indole.
The first three steps are straight from the publication quoted by Nicodem. It can also be found as patent US4643769.
The sulfonate is hydrolised with KOH to the benzaldehyde potassium salt, and further treatet with dimethylsulfate to deprotonate the salt... or what?
:S I'm tired and can't think of the reaction mechanism right now.
All entries in TIHKAL with phenolic indoles, none seems to mention anything about the secondary nitrogen.
| Quote: | Originally posted by Klute
I think LAH can cause complet removal of the sulfonate to the hydrocarbon, so you would need to remove it between the reaction with oxalyl chloride
and the LAH... |
Great thinking! 
[Edited on 7-8-2008 by Dextrose]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Dextrose
...But if ethers or esters would direct the nitro-group correctly, like a sulfoxide, this would of course be the route of choice.
That would be if the pyrrol-formation is possible in any of the latter two...
[Edited on 7-8-2008 by Dextrose] |
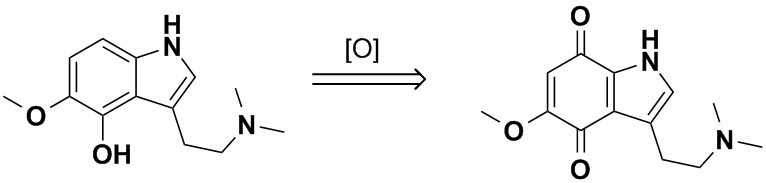
Your figured 4-hydroxy compound would not be very stable with respect to attack by oxygen. By analogy to psilocin found in mushrooms, enzymatic
hydrolysis of psilocycybin gives the free 4-hydroxytryptamine which undergoes easy air oxidation to form a blue quinone derivative
(3-(2-(dimethylamino)ethyl)-1H-indole-4,7-dione). This is the 'bluing' reaction that psychedelic mushroom collectors use to confirm the identity of
their finds. You would either have to acetylate it or protect it as the phosphate ester as in the original Hoffmann psilocybin synthesis. Otherwise
your scheme would likely result only in pretty blue junk, with the 5-methoxy group only helping to speed up the oxidation to the quinone.
[Edited on 9-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
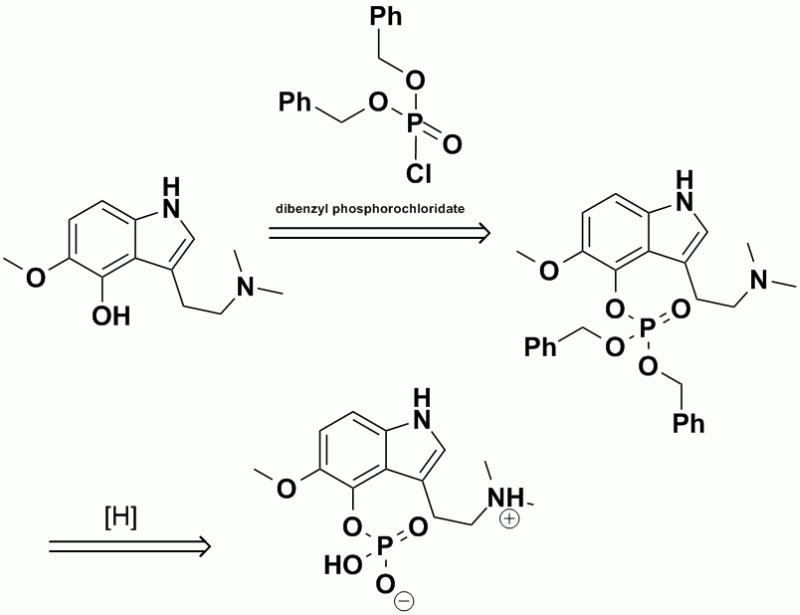
IIRC, this is the final part of the scheme that Hoffman used to prepare psilocybin. The 4-hydroxyl group was esterified with
dibenzylphosphorochloridate, followed by hydrogenolysis of the 2 benzyl groups to produce the final phosphate ester, which is shown in the ChemDraw as
the zwitterion.
Resized to 800x615 pixels with Irfanview.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Due to the problems with nitration it may be best to use the Hemetsberger reaction which is discussed in the J. Braz. Chem. Soc. article in the first post and in the attached paper producing the
4-BnO-5-MeO-6-Me-Indole ester where you want the 4-BnO-5-MeO-Indole ester. This can then be decarboxylated as described in the J. Braz. Chem. Soc.
paper.
So, protect the -OH of ortho-vanillin with PhCH2Cl and react with methyl azidoacetate as described in the attached paper to get the indole precursor
in pretty good yields.
Ethyl azidoacetate (which appears to work just as well) can be made from ethyl acetate ---P/Br2---> ethyl bromoacetate ---NaN3---> ethyl
azidoacetate (See the J. Braz. Chem. Soc. paper for this last transformation).
Have a look at Sundberg's "Indoles" book as well which might be of interest and has been kindly provided by fractional in the References section.
Forming the 4-OAc product can be accomplished in one-pot whilst debenzylating, though it should be subjectively different from the 4-OH analog, so try
both! Best of luck. Here's the paper:
Attachment: 4-BnO-5-MeO-6-Me-Indole from Hemetsberger Reaction.pdf (1.2MB)
This file has been downloaded 1187 times
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Thank you once again, Ritter and Sonogashira...
I have been looking at nearly every indole synthesis i could think of (7-8 kinds) for an alternative...
I hope i don't start dreaming about indoles, waking up in my bed, screaming! 
Sundbergs book would definitly be a good read, but it seems the forum is password protected.
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
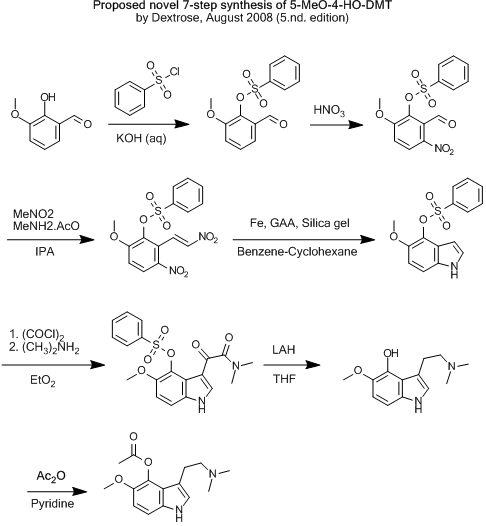
| Quote: | Originally posted by Klute
I would rather go through the sulfonate, I'm pretty sure the benzyl ether will get removed by LAH, and nitrated by HNO3 (you are nitrating a aldheyde,
which is more desactivated that the benzyl ether..). You could just aswell leave the sulfonate on until the LAH reduction, no need of removing it
straight away and thne acylating. I think LAH can cause complet removal of the sulfonate to the hydrocarbon, so you would need to remove it between
the reaction with oxalyl chloride and the LAH... |
Did you mean "so you would not need to remove it between the reaction with oxalyl chloride and the LAH" ?
If so... maybe one could get away with this?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm very surprised. I was sure LAH reduction of aryl sulfonates caused complete deoxygenation to the hydrocarbon.
But when checking what protective groups could be ideal here, in the excellent book "Protective Groups in Organic Synthesis Greene, T. and Wuts, P. "
(a must-read!) , i see:
| Quote: |
Sulfonates
An aryl methane- or toluenesulfonate ester is stable to reduction with lithium aluminium hydride, to the acidic conditions used for nitration of an
aromatic ring
(HNO3/HOAc), and to the high temperatures (200-250°) of an Ullmann
reaction. Aryl sulfonate esters, formed by reaction of a phenol with a sulfonyl
chloride in pyridine or aqueous sodium hydroxide, are cleaved by warming in
aqueous sodium hydroxide. |
So it seems TsO- would be the ideal protective group, and you could keep it even through the LAH reduction. Just hydrolyze off after, and acetylate.
You might want to check if some kind of transesterification can be possible, doing this in one step.
I would again suggest using the TEA or pyridine in DCM sulfonation procedure rather than the crude aq. NaOH or KOH method here.
You might want to check if you can find some mor einfo on the deprotection of an molecuel similar to this one, to insure reflux in basic solution
can't be detrimental. If someone can acces the Bull. Soc. Chem. articles mentionned above, I will translate them.
I can't find the article where I saw LAH used to desoxygenate phenols via their mesylates, only one version using Pd/C and Et2NH, so I'll give myself
a big slap in the head (done) (seriously  ). ).
[Edited on 12-8-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
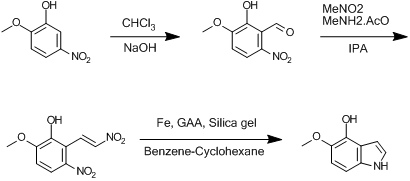
Could it be more possible that a Reimer-Tiemann formylation of 2-methoxy-5-nitrophenol would yield the wanted aldehyde (and indole) ?
[Edited on 18-8-2008 by Dextrose]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Riemmer Tiemann is a bit of a messy, low yileding procedure, and not really suitable for expensive starting substartes... I would definatively use
the Mg-mediated formylation of phenols in such a case, routine >80% yields on various phenols.
But it seems this reaction wouldn't apply well to your substrate, the o-MeO group is known to inhibit mg-mediated formylation (Aldred et al, J. CHEM.
SOC. PERKIN TRANS. 1, 1823-1831 (1994), availble at the ref forum).
Maybe a vilsmeyer formylation of the hydroxy-protected substrate might be better yielding. In any case, I would look for something else than a RT,
which is messy, low yielding and hardly regioselective...
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
meme
Harmless

Posts: 23
Registered: 2-4-2005
Member Is Offline
Mood: No Mood
|
|
Cheap and dirty
OK, I'm a little tired so with that caveat . . .
This all looks pretty hard. I think I might have a DIRTY method that would suck on the first step, but would be cheap and only 5 steps.
"Interestingly, homolytic attack by *OH on the indole nucleus affords mainly 4-hydroxyindole.
M. Julia and F. Ricalens, Contem. Rend. (C) 275, 613 (1972). "
Taken from:
Newkone, George R. Contemporary heterocyclic chemestry. John Wiley and Sons, Inc. 1982. p 76.
Maybe one could hydroxylate 5-meo-indole first, and get a mess of hydroxylated crap, which would actually have some gold (like 5-meo-7-ho). I don't
like dirty reaction like this, but it would be ghetto  It would take some
cleaning but I think DCVC (Dry Column Vacuum Chromatography) might be an easier way to clean up all the isomers, certainly cheaper than some other
methods. It would take some
cleaning but I think DCVC (Dry Column Vacuum Chromatography) might be an easier way to clean up all the isomers, certainly cheaper than some other
methods.
OK, five steps (if the first one works):
1. 5-MeO-Indole --+ 4-HO-5-MeO-Indole
2. 4-HO-5-MeO-Indole --+ 4-AcO-5-MeO-Indole
3. 4-AcO-5-MeO-Indole --+ 4-AcO-5-MeO-Tryptophol
4. 4-Aco-5-MeO-Tryptophol --+ 4-AcO-5-MeO-Indole-3-ethylbromide
5. 4-AcO-5-MeO-indole-3-ethylbromide --+ 4 aco 5meo dmt
I just wanted to throw this out while I try and think of other, cleaner, methods.
Also:
Julia, M. and Ricalens, F., Hydroxylation of N,N-Dimethyltryptamine: factors governing orientation C R Acad Sci Ser C 275, 613, 1972
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
meme's suggestion is interesting, but why not start from 5-MeO-DMT instead?
Here's a paper detailing the synthesis of 4-AcO-5-MeO-Indole via the nitroalkene route:
[Edited on 20-8-2008 by sonogashira]
Attachment: 4-AcO-5-MeO-Indole Synthesis.pdf (494kB)
This file has been downloaded 2368 times
|
|
|
meme
Harmless

Posts: 23
Registered: 2-4-2005
Member Is Offline
Mood: No Mood
|
|
My thoughts were that 5meoDMT is more costly, and that I'd rather have loss at the beginning of a synthesis.
But it would be a two step-synthesis, come to think of it, and I bet a Chinese firm if you shopped around would get this to you at a very good price.
Heck, you could even extract it, purify the DMT, beta-carbolines, and bufotenine out of it if there was any, and start there. If one used a phalaris
known to produce 5meo only, it would be free almost to make some. Yields would be awful, I bet, it is the most low tech thing ever! Grow grass,
extract, free radical reaction *OH, and cleanup (hardest step by 1000 fold).
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
How many times are you gonna attach the picture of same route?  Imo, to make
4-subst indoles the best is to go via nitrene insertion as key step. i.e alkyl azidoacetate + aldehyde, cyclisation/nitrene insertion of the
condensation product, hydrolysis, decarboxylation... Imo, to make
4-subst indoles the best is to go via nitrene insertion as key step. i.e alkyl azidoacetate + aldehyde, cyclisation/nitrene insertion of the
condensation product, hydrolysis, decarboxylation...
|
|
|
| Pages:
1
2
3
4 |