Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Online
|
|
why you shouldn't touch homemade salts
I know chemicals can be toxic and we should always wear gloves (even if they're expensive and annoying)
But doing chemistry isn't like anything else we chemists are more likely to get mercury poisoning than Individuals with dental amalgam fillings simply
because WE MAKE EVERYTHING SOLUBLE
I used my imagination to make this I know this sounds stupid but please bare with me 
two guys bought a beryllium bronze Adjustable wrench they thought it's pure copper okay? the first one didn't care actually
The first guy is a plumber, he used the wrench on a daily basis with no issues
The second guy is an amateur chemist '-' and wanted to make copper sulfate and like a normal chemist he dissolved the whole thing in sulfuric nitric
acid mixture two days later he touched the pretty CUSO4 5H2O crystals without gloves because you know he can, now he's dead  (the good doctor voice) (the good doctor voice)
In solid form and as finished objects, beryllium copper presents no known health hazard But the poor chemist is freaking dead right now! Are YoU
happy?
Anyways  that's why we should always expect the worst and use water heavy metals
test strips before we extract metals and potassium rhodizonate for lead etc that's why we should always expect the worst and use water heavy metals
test strips before we extract metals and potassium rhodizonate for lead etc
Please feel free to post any thoughts for testing 6 toxic metals:
thallium mercury cadmium beryllium arsenic lead
Nickel cobalt and chromium aren't toxic as these metals so I didn't write them and the lethal dose is higher than few micrograms

[Edited on 6-11-2020 by Fluorite]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
"Others in the metal recycling industry are just now learning of the risks. Computers, telephones and other scrap with bits of beryllium are recycled
for the gold, silver and copper inside. When those items are burned, crushed and melted, beryllium dust or fumes may be released."
"In the United States, several workers are known to have contracted beryllium disease this way. Toronto-based Noranda Inc., one of the world's largest
recyclers of electronics, reports that 13 workers in its Canadian facilities have been diagnosed with beryllium disease recently and 30 show blood
abnormalities, including a company nurse."
https://www.chicagotribune.com/news/ct-xpm-2001-07-29-010729...
|
|
|
Metallophile
Hazard to Self
 
Posts: 87
Registered: 23-3-2018
Member Is Offline
Mood: No Mood
|
|
This is interesting, and somewhat frightening. I have a small machine shop, and we do run some BeCu strip. We only do shearing and forming though, no
grinding. I've always assumed this meant we were not at risk, as the smallest particles created would be "chips" on the order of the material
thickness. We recycle all the scrap, and I wonder if there's a way for me to (safely) get some Beryllium out of there? I believe the alloy we use is
4% Be, which is not insignificant.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
You can separate copper and beryllium sulfates quite easily. Just heat them to get off all water of hydratation. While anhydrous CuSO4 is soluble in
water, anhydrous BeSO4 don't. So you can dissolve solid, filter it and let evaporate to get pure copper sulfate pentahydrate.
Test for:
Tl - thallium forms insoluble chloride, yellow chromate and sulfate is soluble (this is for differentiation from lead). I also read that Tl+ form red
complex with rhodamine B (Sb also forms red complex)
Pb - lead forms insoluble chloride, yellow chromate, insoluble sulfate
Hg - SnCl2 firstly reduce Hg2+ in to white Hg2Cl2. Excess of reagent reduce Hg2Cl2 in to elementar mercury. Hg also forms insoluble Cu2[HgI4] and
Ag2[HgI4] which are thermochromic, see this.
Cd - Forms insoluble yellow sulfide insoluble in excess of reagent
As - Yellow sulfides (As2S3, As2S5) soluble in excess of reagent, pentavalent arsenic with acidified ammonium heptamolybdate give yellow precipitate
(this precipitate also form phosphates and silicates).
Be - Unlike other alkaline earth metals forms soluble sulfate (along with Mg) and fluoride
But this is just simplified, it also depends on other ions in solution.
Btw. Arsenic (respectively its the most common form grey arsenic) is actually semimetal. But also exists nonmetallic (yellow arsenic As4) and metallic
(black arsenic) forms, which are not that common.
|
|
|
Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Online
|
|
Thank you Bedlasky so much
Please guy be careful
Maybe we can separate copper from any metal by washing cuprous chloride with a lot of water, adding ammonia, crystallization 4 times or more and also
if copper sulfide isn't soluble in polysulfide we can make it and all metal sulfides like mercury and cadmium should dissolve
Idk about beryllium but as far as I know it's rare
[Edited on 6-11-2020 by Fluorite]
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Fluorite, this is an awfully convoluted story you’ve come up with to explain what is essentially an imaginary problem. Nobody in their right mind
would use a Cu-Be tool as a source of copper. It’s way more expensive than copper electrical wire, which is readily available and either cheap or
free if you can find it as scrap, and very pure copper.
|
|
|
Texium
|
Thread Moved
6-11-2020 at 10:41 |
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The opening story isn't plausible.
A beryllium bronze wrench would be a very expensive way to buy copper- about £110 on ebay.
It would also be a stupid choice- since it's thick, it would take a long time to dissolve.
And someone who does not notice that it's labeleled as Be Cu isn't an amatuer chemist, they are an idiot.
And you don't get Be poisoning by skin contact with some impure copper sulphate.
So, how about not giving ammunition to the homophobes?
|
|
|
Metallophile
Hazard to Self
 
Posts: 87
Registered: 23-3-2018
Member Is Offline
Mood: No Mood
|
|
I was thinking of it more as a source of Beryllium. We generate 10s of pounds of the scrap metal, and I only get $1.50/# for it from the scrap dealer.
The alloy is 2%, not 4% like I remembered. But that should still be enough for a small sample for my collection.
|
|
|
Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Online
|
|
Haha I know this can't happen I just wanted to say how can dissolving pretty safe stuff can be deadly but thanks unionised for help
Btw what does 'thread moved'. Mean?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It means you put it in the wrong place and one of the mods has moved it.
Quote: Originally posted by Fluorite  | Haha I know this can't happen I just wanted to say how can dissolving pretty safe stuff can be deadly but thanks unionised for help
|
Did you think that through?
By dissolving deadly beryllium in copper, you end up with something relatively safe.
[Edited on 6-11-20 by unionised]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
What is the LD50 for beryllium and it's compounds? It's easier to picture how toxic it is, can it be accidentally exposed to or does it need actual
consumption? 35 micrograms on average is found on human body anyways.
I believe one can obtain beryllium in much more convenient form than as alloyed wrenches. 0.5-3% content is technically a joke as a source. It should
be sourcable because it has wide variety of uses in pure form. Other thing is, what to do with it, if it's exceedingly toxic and performs same
functions in chemistry as less toxic alternatives?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
That claim is debatable at most.
In fact, I've seen a study that compares the likelihood of chemists for job related diseases like to other jobs, I think I've found it at kilomentors
blog, and the result was there isn't any such correlation.
In general, not just for mercury poisoning, but also cancer types related to chemical exposure, etc.
As a sidenote, I would even speculate that dentists are the major group affected by mercury poisoning and not chemists.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I think I saw that discussion and it clearly stated that chemists show no elevated risk of getting toxicity/cancer related health issues. I believe
this is the case, because they might get occasional sniffs from known carcinogens, but the exposure is so minute and brief it has no effect. People
who expose occupationally to these things may do it 8 hours a day for decades. I have concluded that taking a sniff of toluene few times in your life
is practically of no risk compared to soaking your hands in toluene solvents in industry on daily basis.
The risk of acute poisoning exists of course and it can be 100% higher compared to people who do not handle such things. For example, risk of getting
dimethylmercury poisoning is up to 100% if you handle it, and 0% if you don't.
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
I spent over 7 years working with beryllium copper alloy building RF cable assemblies for the aerospace and defense industries. I came into contact
with parts, chips, and sanding dust on a daily basis, even impaled a few fingers with chips and small diameter parts. No health issues experienced by
me nor any coworkers while I was there nor any I am aware of in the twenty years since. Basic housekeeping and dust masks are all that is needed.
Fluorite, you may want to consider changing your username to Chicken Little. Your warnings may be based on good intentions but they seem misplaced and
exaggerated. Besides, you know what they say about road construction and good intentions...
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by OldNubbins  | I spent over 7 years working with beryllium copper alloy building RF cable assemblies for the aerospace and defense industries. I came into contact
with parts, chips, and sanding dust on a daily basis, even impaled a few fingers with chips and small diameter parts. No health issues experienced by
me nor any coworkers while I was there nor any I am aware of in the twenty years since. Basic housekeeping and dust masks are all that is needed.
Fluorite, you may want to consider changing your username to Chicken Little. Your warnings may be based on good intentions but they seem misplaced and
exaggerated. Besides, you know what they say about road construction and good intentions... |
There is a difference in making contact with metal in elemental form, and its ions in the form of its salt.
For example, you coud've even drank elemental mercury and nothing very serious would've happened, but if you drank mercury salt solution you probably
would be poisoned as hell or dead.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mackolol  |
For example, you coud've even drank elemental mercury and nothing very serious would've happened, but if you drank mercury salt solution you probably
would be poisoned as hell or dead. |
I could have sworn I'd posted this already, but can't find it now.
| Quote: |
A 21-year-old dental assistant attempted suicide by injecting 10 ml (135 g) of elemental mercury (quicksilver) intravenously. She presented to the
emergency room with tachypnea, a dry cough, and bloody sputum. While breathing room air, she had a partial pressure of oxygen of 86 mm Hg. A chest
radiograph showed that the mercury was distributed in the lungs in a vascular pattern that was more pronounced at the bases. The patient was
discharged after one week, with improvement in her pulmonary symptoms. Oral chelation therapy with dimercaprol was given for nine months, until the
patient stopped the treatment; the urinary mercury level did not change during this period. At follow-up at 10 months, she was healthy, with none of
the renal, gastrointestinal, or neurologic effects that can result from the oxidation of mercury in the blood and consequent exposure of these organ
systems. The abnormalities on the chest radiograph were still apparent. Although these abnormalities are striking, the absence of clinical toxicity in
this patient illustrates the differences in the acute and chronic effects of exposure to elemental mercury, inorganic mercury (e.g., mercuric
chloride), and organic mercury (e.g., dimethylmercury). Inorganic and organic mercury are much more toxic than elemental mercury; for example, a dose
of 400 mg of mercury in the form of dimethylmercury is usually lethal.
|
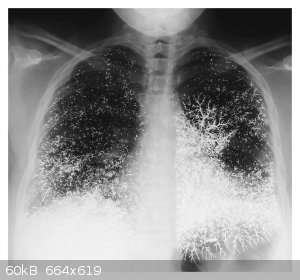
Elemental Mercury Embolism to the Lung. Francisco Gutiérrez and Lucio Leon. N Engl J Med 2000; 342:1791 DOI: 10.1056/NEJM200006153422405
In looking this up, I found out that shooting up with quicksilver is not as rare as you might think (?!)
| Quote: |
A 15 year old previously healthy girl presented with an acute febrile illness with a generalized maculopapular skin rash for 3 days with a preceding
history of self-injection of mercury to both her forearms. This was an imitating experimental act influenced by a movie and she was mentally sound.
Very high whole blood mercury levels, x-rays of the forearms and histology confirmed mercury poisoning.
|
https://bmcresnotes.biomedcentral.com/articles/10.1186/s1310...
| Quote: |
A 20-year-old man presented with diffuse chest pain for three days and moderate grade fever for one day with hemoptysis. About three months earlier he
had injected approximately 5 ml of elemental mercury subcutaneously into his left forearm; in addition, seven days prior to presentation he had
injected approximately 8-10 ml of elemental mercury (obtained from a sphygmomanometer) intravenously.
|
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2890926/
| Quote: |
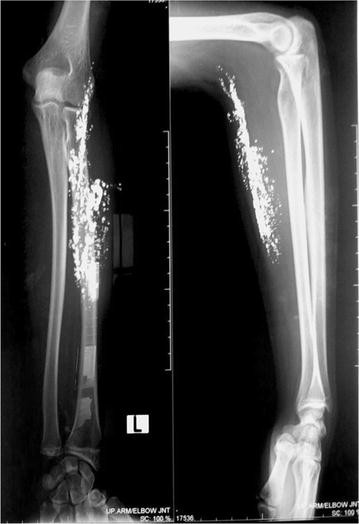
Case 1: A 22 year-old trainee in alternative medicine was admitted in our hospital on 8th February 2005 with a history of intravenous injection of
elemental mercury in both hands [Figures [Figures 1 1–4]. On examination, the patient was found to have fever, tachycardia, and inflammation of
both hands extending up to the forearm. The patient had had an incision and drainage for a supposed abcess in another hospital. Radiography of both
hands showed a radio-opaque shadow extending from the tip of the ring finger to the lower third of the radius and ulna on the left hand, and from the
PIP joint of the ring finger to the wrist joint of the right hand. The patient left against medical advice before any further investigations or
surgical procedures. He was later admitted to a private nursing home and it was reported that he died within 48 h of admission. No further information
was available from the relatives except that he had taken many other injections in addition to the mercury, and that he had been doing this for more
than two years.
|

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2740532/
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Oh my god, what the heck, how, why, what?
That is scary looking!
I didn't know that mercury can be put into a vein though, and imagining this makes me feel very uncomfortable.
And how would they extract it, would it flow out of the depots it apparently formed or what?
I never even knew that such a thing is possible, nor that some people would even do that, first time I ever hear and see something like this!
Its quite the shock! 
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Fascinating that the patient with the mercury filled lungs apperantly is doing fine!
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I have heard about injecting mercury intravenously too, but damn man what would be the feeling of injecting something so fucking dense into your vein,
for sure the hand would feel quite heavy hehe.
My friend knows a person, totally addicted to IV opioids and when she was boring and had no dope, she would just inject distilled water into her
veins, because "she liked the feeling". I better not tell her about mercury though 
Apparently, pharaohs in ancient egypt were drinking elemental mercury, as they would think that it's the superior elixir that couses immortality. They
seemed to function well, at least for few years...
I wonder how does the body excrete elemental mercury that's in digestive system. Does it go along with feces?
[Edited on 7-11-2020 by mackolol]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yes, it simply gets excreted along with all the other waste.
Thats why drinking a kilo or two of mercury was an early remedy against constipation 
This dense stuff just forces itself out, it literally falls out of your guts I think.
I wonder if such a dense weight being "dropped" ever destroyed a porcellain throne in the process?
The mental picture sure is quite some fun for me 
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Emperors and other madmen had a fascination to mercury all over the history and they sipped it thinking it was an elixir of youth or bringer of
eternal life. Most of it just passes through, but some will be absorbed, and the toxicity of mercury is in the minute amounts, so you're *ked anyways.
|
|
|