neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
A general question on high pressure piping
Off in the distant future, I have a plan for building a supercritical carbon dioxide extraction vessel. The problem is that this involves very high
pressures and moderately high temperatures (1100 psi, 32*C). My plan is simple: get a threaded pipe of ~1.5 inches diameter, put removable screw-on
caps on both ends, and weld a pressure release valve into one of the caps.
Is this feasible? How easy to obtain are these pipes and caps? Will the threading hold the caps in place with this much pressure pushing them out? Are
these materials usually rated for these temperatures? I have heard that some steels weaken even at relatively low temperatures, are temperatures like
these workable? How dangerous would this be?
I have no experience with building things like this, so any insight is appreciated.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
Aren't these the normal pressures encountered with normal CO2? I've used regular 1/8" steel and brass plumbing fittings to refill smaller CO2 tanks
and to hook up regulators. You could also use steel brake line tubing, as it's working pressure is 900psi. I always 'test' my hookups with a slow
pressure buildup while standing behind something massive...just in case. I don't think you have to worry about steel at 32*C. If you wish to be
really safe, fill the pipes first with water , (brake fluid, hydraulic oil, motor oil) and pressurize it to 1500 psi with a small displacement
pressure testing pump. Since water doesn't compress very much, a failure won't be as noisy and dangerous as that much compressed gas. I welded up a 40
gallon rolled steel tank for a travel trailer and then tested it with a plumber's pressure test pump to 500 psi. The small leaks were soon re-welded
and it served for years until rust caused it to leak. You could even scavenge an automotive master cylinder with reservoir and use it to pressure
test the apparatus.
[Edited on 24-2-2006 by Mr. Wizard]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Definetly hydrotest, to double operating pressure at least.
(Common BBQ tanks are rated for 100-200PSI regular use, 500PSI design, and bursting typically over 800PSI, as I recall. Yeah, that's for tanks in use
by the *general public*, but nonetheless gives you some idea of a good safety margin in these matters.)
Strength isn't a problem until at least 200C, and that's for hardened steels. You definetly don't want to be using those, anyway. Something softer
that bends and splits rather than shatters.
As I recall, typical steels start creeping by 400C or so.
If you'd like an alloy recommendation I'd think 4130 worked to the right degree would be very nice here: high strength, yet high elongation, e.g. 4130.
Tim
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Neutrino,
It's best to always use Seamless Piping, this is Piping that has been Extruded out of one Piece. The cheap stuff, contains a Weld in the longitudinal
plane of the Piping. On the outside, it's often hard to see, especially if the Pipe has been polished. On the inside however, a slight discoloration
can be seen where the Weld was made, due to the high temperature that was involved. But this too, can at times be relatively hard to see. But on the
other hand, ~80 Bar is not exceptionally high, even for certain Seemed Piping standards (but not that household Gas Piping). But it's best to play
this safe. People have been killed by just pumping up there Car Tire, and even more so with Lorry Tires at a pressures far below 80 Bar. Just
remember, that the force on your End Caps can be as much as 0.9 Tons (900 Kg = ~2000 Pounds). More energy than the average small caliber Bullet has,
and a killer too. So please play safe on these End Caps. They can however be Threaded, but it may be better (safer) to have them Welded on by an
Experienced Welder.
You have mentioned 32 Degrees Celsius, so temperature will have no effect, for these Pipes are tested within this range, so that the factory specs
should hold up quit well.
Gas Cylinders are usually specified till 50 Degrees Celsius (with content), which would be a much better choice as a Container. An small empty MIG CO2
Cylinder (~30 cm long and ~7.5 cm diameter) should work out just fine, and filled, you can buy them for about $10 - $15. They are Threaded too.
You can also check out your local Scrap Yard, and you will be able to find Gas Cylinders in all sizes. Hell, maybe even the Air Buffer Cylinders of an
old Car may work out (the French stuff, you know). These Scrap Yards are often filled with this kind of Pneumatic Braking and Buffering stuff. Diesel
Motor Pumps and Piping even piss on 80 Bar as if it were Peanuts. No more Threading to be done, it's all there, waiting for Neutrino to pick it up, to
use it, and abuse it.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Yes, the water test. I’d forgotten about that one. I’ll make sure to use it.
The main problem is still the end caps. At least one must be removable for loading/unloading of the material to be extracted. The energy stored within
them is also a little scary to think about. Are there any other alternatives?
How easy to get are threaded pipes and caps? I’ve never really looked around for these.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Neutrino I think most high pressure laboratory and industrial work is done with thick walled stainless steel and flanged fittings IMO. I once
wandered down the hall of a nearby university and snooped into the rooms where the graduate students had their projects set up. I saw 2 or 3 really
beefy looking stainless steel vessels. I didn't get a real close look as I was just snooping and not on a guided tour but I presume these were high
pressure reactors.
Mostly to avoid leaks we nearly always used flanged or welded connections at work. Threads introduce weakness as they obviously decrease wall
thickness.
If you must stay with threads look for fittings rated as 150 lb, 3000 lb, or whatever. This rating means they are good for saturated steam at 150
psi, or 3000 psi, etc. At your lower temperature of 32C they would be good for even higher pressure. This higher pressure will be available from the
supplier or from a piping handbook.
Industrial suppliers will have these pipes and fittings. The pipes will be in the higher "schedule" numbers. Normal piping is schedule 40. But pipe
is available in schedule 80 and higher, which just means higher wall thickness. A search on Google should bring up information on these beefy pipes
and fittings.
This website shows a 1.5" 3000 lb cap in forged steel for $6.86: http://www.pipefittings.net/
My reference shows that 1.5" iron pipe is available in schedule 40, 80, 160, and XXS. Wall thicknesses are 0.145", 0.200", 0.281", and 0.400",
respectively.
Ref: "Flow of Fluids Through Valves, Fittings, and Pipe," by Crane (Technical Paper No. 410)
There is also a formula in Perry's Chemical Engineers' Handbook that will allow calculation of how much internal pressure the pipe will withstand,
with or without threads.
[Edited on 23-2-2006 by Magpie]
[Edited on 23-2-2006 by Magpie]
[Edited on 23-2-2006 by Magpie]
[Edited on 23-2-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I know exactly what you are talking about Magpie, I'm actually doing a project that uses quite a bit of Swagelok 316 stainless and unless neutrino has
several thousand dollars to spend (some of the valves are in the hundreds of dollars per piece) I don't think stainless would be an option. Hmm only
1500 psi eh? If anything, I'd suggest you contact someone who sells paint balling equipment; one shop near by has some nice fittings rated for 2000
psi for about 1\8th the price you'd pay if you bought from Matheson TriGas, Swagelok, or any other "special apparatus" supplier. Those won't be in
your specified 1.5" diameter, maybe .5".
I have no doubt that the threading will hold the pressure, as Magpie mentioned, the flange-type work nicely (more or less tighten down a nut until the
flange is squeezed on). I don't know if I'd weld it shut. Perhaps incorporate some sort of blow-out valve as a release if something goes wrong.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, Fleaker, the chemical species, temperature, pressure, velocities, residence time, etc, as always, will dictate your allowable choices of
materials of construction. For just CO2 I don't know why forged steel wouldn't work. My example shows this material to be reasonably priced. But
once you start adding solutes then those and the reaction products will influence your material allowables as well.
One of my long term projects is to build a "maintainable" iron retort. I will probably end up using black iron or forged steel fittings. I want it
to have a flanged connection at the widest part of the vessel so that I can open it up for inspection and cleaning. I will likely also incorporate
screwed connections or welded (weld neck) connections or even weld the connection after screwing together. There are many ways to do this. One has
to look for the cheapest way that still meets your criteria for performance, maintainence, and above all safety.
I agree with these recommendations already made:
A hydrostatic test at 1.5 times the anticipated operating pressure. It is also good practice to have a saftey relief device.
As you can see this is all much more complicated than just assembling quick-fit. 
[Edited on 23-2-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Actually a small paintball CO2 tank would be ideal for extraction and what not for the pressure/temp the OP needs.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Wet supercritical CO2 is rather corrosive. Unles you are sur that the stuff you extract is really dry this could be a big problem.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
The extraction will be done in the presence of molecular sieves in my aerogel idea. Water should not be a problem. Still, thanks for pointing this
out.
The problem with any kind of tank for this purpose is that you couldn’t get any solid material in or out. If I want a nice big block of aerogel, I
have to be able to put the whole block in and take it out later. The tank is only as useful as its opening. That’s why I want piping. Unless someone
has a better idea...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I understand what you are saying neutrino. That is the feature I want for my retort also. For this I especially like flanged connections. The RF
(raised face) type is nice as you place a gasket of your material choice on this face. You then seal the vessel by tightening the bolts to a
pre-determined torque. The torque required is determined by the internal pressure you require.
Problems with screwed connections:
1) reduction in strength due to loss of wall thickness
2) a corrosion/contaminant site that may become very difficult to open
3) harder to seal with each opening/closing cycle
But they are much cheaper and more available.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Okay, you have me sold on flanges. I never really considered that kind of joint before you brought it up.
How much should I expect to pay for the assembly I described with flanged connections? Let’s say something in the area of 6 " long, 2" diameter with
appropriate end caps/plates/flanges?
edit: There's one more thing I forgot to ask. My planned method of filling this container with liquid CO<sub>2</sub> is to put in some dry
ice, seal it, and let it come to room temperature. Would I have problems with the metal becoming brittle at these low temperatures?
[Edited on 25-2-2006 by neutrino]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
As I recall, stainless steel does not suffer from cold embrittlement.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
To keep the walls from getting cold, don't let the dry ice touch the walls. Just suspend it inside the container. Keep the walls warm with a few
wraps of 1/4" copper line flowing tap water, or some other moderate heat source.
Someone mentioned the corrosion with wet CO2, which is a real problem. All commercial CO2 tanks are supposed to be checked for this every 5 years.
Getting dry ice into a container without carrying a layer of water frost on it might be difficult.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Will you want these (optional):
1) pressure letdown port w/ball valve*?
2) pressure gage?
3) safety relief device?
*Pressure could be dropped by just "cracking" open the flange when done with the extraction.
Also we need to know:
1) your vessel material choice?
2) your gasket material choice?
Flanges may have to be welded on to the pipe if not available with screwed ends. I would try to go with a thick-walled pipe and then get all threaded
fittings. That way you won't have to hire any welding. (I'm presuming you are not already a certified welder  .) .)
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
For aerogels, the pressure has to be released very slowly. Would it be cheaper to buy something with a valve built in or to weld one to a cap myself?
I would probably want ordinary steel for the construction. What kind of gasket would be best for supercritical CO<sub>2</sub>? This is a
very powerful solvent, so something very chemically resistant must be used. You probably have more experience with these gaskets than I do. Maybe PTFE
or PFA... Although PTFE creeps under high pressures… Maybe a soft metal like lead?
You’re starting to confuse me on flange vs. thread here. First you praise one then the other…?
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Neutrino,
I can be short on this one, ... Teflon (PTFE) Gaskets. Just let it creep, the better will be the seal. Containing the creep within boundaries, is the
trick to be used, ... and the solution.
[Edited on 25-2-2006 by Lambda]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Sorry for the confusion neutrino. I'll try to explain it better:
The 2 mating flange pieces must each be permanently attached to separate pieces of the pipe body somehow. This can be done via welding or screwed
ends. The screwed ends, once set, would never be opened again.
In either case, the operating opening for your extractor would be a flanged connection.
Teflon (PTFE) is probably a good choice for the gasket but I have no experience with containing liquid CO2. A call to a vendor such as Garlock would
quickly get a recommendation IMO. Or look at their website.
For my work with ammonia (previously reported here) I used
a 1/8" stainless steel ball valve as an isolation valve. Then I had a 1/8" stainless needle valve to allow a slow transfer of ammonia gas. The ball
valve was about $10 but the needle valve was over $50. So it just depends on what you require.
Packing/seats in the valve must also be considered. I think mine are HDPE.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here is the result of a calculation for the maximum working pressure, P, of a nominal 2" steel schedule 80 seamless pipe with screwed fittings, using
the formula in Perry's Chemical Engineers' Handbook, 4th ed.
Assumptions:
1. D = 2.375" (pipe outside diameter)
2. t = 0.218" (nominal wall thickness)
3. mill tolerance (12.5%) = 0.0273"
4. thread depth = 0.085"
5. corrosion allowance = 0. 025"
6. S = 16,000 psi (allowable stress)
7. Y = 0.4 (coefficient for steel)
8. E = 1 (longitudinal weld joint factor for seamless)
9. C = "3" + "4" + "5"
P = 2(t- C)(SE + PY)/D
P = 2(0.218" - 0.1373")(16,000 psi + 0.4P)/2.375"
P = 1118 psi
So by this calculation this pipe would be OK for your intended service. The corrosion allowance is likely more than adequate. If not I think you have
the wrong material.
There is a fairly decent safety factor built into this formula in the stress allowance of only 16,000 psi. That factor would be about 2.
Keep in mind that I'm only a chemical engineer and this is dabling in the black art of mechanical engineering. 
Edit: The attached photo shows a sketch of what this pressure vessel might look like.
[Edited on 26-2-2006 by Magpie]
Delete the word "screwed" from blind flange on the photo.
[Edited on 26-2-2006 by Magpie]
[Edited on 26-2-2006 by Magpie]
[Edited on 26-2-2006 by Magpie]
[Edited on 30-1-2007 by chemoleo]
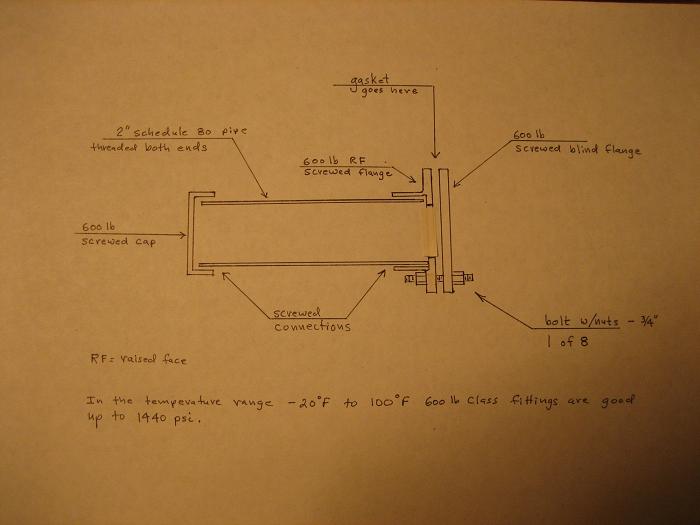
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|