| Pages:
1
2
3
4
5 |
woelen
Super Administrator
        
Posts: 8003
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
MsSO4 is very pale pink, a most delicate color. Probably your material contains some iron. If you have a soluble thiocyanate salt, you could try
adding a pinch of your yellow salt to a drop of fairly concentrated thiocyanate solution. If the impurity indeed is iron, then the drop will turn
blood red, even when only a tiny amount of iron is present in your sample. Manganese(II) does not give a strongly colored complex with thiocyanate
ion.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
This thread is getting popular, should it be pinned?
|
|
|
Chem Science
Hazard to Others
  
Posts: 122
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Amazing that PhNHNH2.HCl Very nice crystals !! Plis post your synthesis  
|
|
|
Tsjerk
International Hazard
    
Posts: 3028
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I think I can make a nice photo of MnCl2. Mine is nice and pink.
Edit; Mine could also be the sulfate... I haven't seen the bottle in years, but I know where it should be.
Quote: Originally posted by DrScrabs  | Sorry, not the best quality of the image. But the PhNHNH2.HCl is triple recrystallized from EtOH and got a nice treat with activated C.
Made by the NaSO3/SO2 reduction(SnCl2 is bs here IMHO), If there is some interest I can post the synthesis next time I make some  |
Is yours very fluffy? I have a commercially bought sample and it is very voluptuous. Like 0.3 grams filling a cubic centimeter.
[Edited on 6-4-2019 by Tsjerk]
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Quote: Originally posted by Tsjerk  |
Is yours very fluffy? I have a commercially bought sample and it is very voluptuous. Like 0.3 grams filling a cubic centimeter.
|
Yes it is.
|
|
|
woelen
Super Administrator
        
Posts: 8003
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Here follow pictures of a few compounds I made at home:
This is potassium, I made according to POK's method, first posted on the German forum Versuchschemie.de. This forum unfortunately does not exist
anymore.

The following is KCrO3Cl, I made from K2Cr2O7 and conc. HCl.

The next one is N(CH3)4ICl4. Normally, salts of the complex ion ICl4(-) are not very stable and this compound easily loses ICl3 so that a simple
chloride remains behind. With the big cation N(CH3)4(+), however, it forms a stable salt.

The final one is KVO4 (the yellow stuff) and KVO3 (the white stuff), made from V2O5 and KOH and H2O2.

|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Crude phenylhydrazine sulfate, thread coming tomorrow 

|
|
|
Tsjerk
International Hazard
    
Posts: 3028
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Nice! Do you know why it is reddish?
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Oxidation maybe.

Iodine from NaI + KHSO5, molten under H2SO4, forms a nice on cooling crystalline layer without I2 fumes!
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
do you have any problems with sulphuric acid bubbles in the solid I2 mass?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
No, it went flawless! The I2 obtained by the reacrtion was a fine precipitate, washed(dH2O<3°C) on the sinter and, slightly wet transfered to a
beaker filled with a few ml (enough to cover it all) of concentrated(>97%) H2SO4 and heated above the melting point if I2. I2melted and formed a
nice liquid layer under the acid. I stirred with a glass rod occasionally and left it on the hotplate for appx 30 min @melting point.
The I2 layer is without any bubbles containing H2SO4 or sth else(as far as I can tell)!
The crystal structure is "flaky", like flat crystalline layers forming on top of each other.
Edit: The crystalline structures on top of the flat mass on the pictures are from "refluxing" I2 vapour during storage IMHO (storage 10 months+) wich
may be the most interesting in the picture, the bottle has small crystalline deposits of I2 everywhere!
[Edited on 13-4-2019 by DrScrabs]
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Here are some Manganese Chloride I synthesized a few years ago.
There might be a bit of iron contamination, because that was produced from "pottery grade" Manganese Carbonate, and hardware store HCl. But I doubt it
because it has the proper color (I synthesized MnCl2 from regular C-batteries a few years back and it had a orange-ish tint)

--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Bedlasky
International Hazard
    
Posts: 1227
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Hello.
I made some solution of sodium hypomanganate cca 8 months ago. I am really interested in manganese chemistry and I prepared every oxidation state from
II-VI from KMnO4. But hypomanganate is really unstable and rare. This makes him really exotic compound.
I made him by reduction of KMnO4 by Na2S2O3 in 48% NaOH. All reagents was cooled in fridge before reaction because hypomanganates are more stable in
lower temperatures. In to the solution of 48% NaOH I added few drops of 25% solution of Na2S2O3.5H2O (Na2S2O3 isn't dissolve in water, but in 20%
NaOH). Then I added few drops of dilute aqueous solution of KMnO4. Colour of solution has turned in to green by presence of manganate. I returned
solution in to the fridge and after ten minutes solution turned colour in to turquoise by presence of hypomanganate. Solution was little bit cloudy,
because some NaOH in solution crystallized in the fridge before reaction.
On the internet I found that this compund have really interesting formula Na3MnO4.0,25NaOH.12H2O.
I plan to repeat this reaction in future and test how long solution stays turquoise and some redox chemistry with ethanol and acetone.
In the picture is solution of hypomanganate next to solutions of manganate and permanganate for comparison of colour MnO4(n-).
Sorry if my english is bad. I'm trying as much as I can writing correctly.

[Edited on 15-4-2019 by Bedlasky]
[Edited on 15-4-2019 by Bedlasky]
[Edited on 16-4-2019 by Bedlasky]
|
|
|
woelen
Super Administrator
        
Posts: 8003
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Very interesting manganese chemistry. I know the green manganese(VI) and made this myself quite a few times, but the bluish manganese(V) I only knew
from literature. Never found a way to make it myself, all attempts lead to formation of finely divided solid yellowish/brown manganese(IV) compounds
(most likely hydrous forms of MnO2).
|
|
|
j_sum1
Administrator
       
Posts: 6277
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Yes. I have only seen the blue +5 of Mn in photos, and very few of those too. It has been on the list to do. Extremely pretty colour.
Thanks for the reminder.
Edit
Provedure for Mn(V) here. Navigate to Mn oxidation experiments
http://www.explorechem.com/home.html
[Edited on 17-4-2019 by j_sum1]
|
|
|
Bedlasky
International Hazard
    
Posts: 1227
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I made few experiments until it's finally working. First few experiments stopped on manganate. It needs very strong alkaline media and low
temperature.
If you want to try prepare hypomanganate in solid I have read something about this in one document (but it's only in Czech  ). Author made hypomanganate from K2CO3, MnCO3 and dry air (dried over conc. H2SO4) at
800°C. The reaction leasted 60 hours. But hypomanganate wasn't pure - the yield was 56,9% (impurities was MnO2 adn K2MnO4). Hypomanganates is very
water sensitive. Trace of water in the air leads to disproportionation to MnO2 and K2MnO4. ). Author made hypomanganate from K2CO3, MnCO3 and dry air (dried over conc. H2SO4) at
800°C. The reaction leasted 60 hours. But hypomanganate wasn't pure - the yield was 56,9% (impurities was MnO2 adn K2MnO4). Hypomanganates is very
water sensitive. Trace of water in the air leads to disproportionation to MnO2 and K2MnO4.
[Edited on 17-4-2019 by Bedlasky]
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Over the years I have accumulated a few pictures of unusual chemicals. Here are 4 I have taken over the years.
1. is Selenium dioxide crystals.
2. is a selection of phosphorus samples.
3. is Neodymium sulphate.
4. is Praseodymium sulphate.
   
If you're not part of the solution, you're part of the precipitate.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
And of course Europium 3+ in alkali fluorescing under a UV laser.
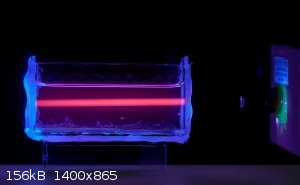
If you're not part of the solution, you're part of the precipitate.
|
|
|
woelen
Super Administrator
        
Posts: 8003
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
All of these samples are very nice, but the white phosphorus and the europium are really outstanding.
My white P is not transparent, it is more like off-white wax. I have seen such pure white P once before, made by Endimion17 some years ago.
I myself did a little experiment and made a few pictures again, which also may be interesting. A small experiment, dissolving some I2 in CCl4, which
gives a nice purple solution. The picture shows a still very dilute solution, which best demonstrates the color.

Finally, I had a very dark purple solution. To this, I added a little amount of red P and swirled for a minute or so. The I2 reacts with the red P
(which was in excess). This leads to formation of the red compound PI3, which also is soluble in CCl4 (but only sparingly so).

I ampouled some of this solution. This solution is quite nasty, it fumes in contact with humid air. These fumes are from HI, formed by reaction of
water vapor in air with PI3, dissolved in CCl4.
I ampouled some PI3, dissolved in CCl4 to make a more permanent display:

Making more PI3 in this way is not easy, because its solubility in CCl4 is not that high. Also the solubility of I2 in CCl4 is not superb.
I also have a small sample of the solid compound (made with CS2 as a solvent which has much better solubility properties) and ampouled this in a 20 ml
ampoule to keep it good. It is very air-sensitive and cannot be kept around in a normal bottle (it is very corrosive, like PCl5, PCl3 and PBr3, and
eats caps and besides that, it gives nasty brown stains on anything nearby). I made the glass ampoule a few days ago and now it is safely stored and
makes a nice display:

|
|
|
woelen
Super Administrator
        
Posts: 8003
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Another nice sample, this one is appr. 25 grams of reagent grade BBr3. This I purchased as shown. I did not ampoule this myself. Making BBr3 at home
is very difficult, boron and Br2 do not react at room temperature. Making this compound requires the use of very high temperatures.

Pure BBr3 is colorless, but commercial samples always contain a little free bromine, giving a brown/orange color.
[Edited on 17-4-19 by woelen]
|
|
|
Bedlasky
International Hazard
    
Posts: 1227
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
The crystals of neodymium and praseodymium sulphate have really nice colours  .
Europium is also very nice. The sample of white phosphorus is very interesting - I've never seen sample like this. .
Europium is also very nice. The sample of white phosphorus is very interesting - I've never seen sample like this.
Purple colour of iodine solution reminds me school labs - determining of partition coefficient. PI3 have nice colour  . .
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | Hypomanganates is very water sensitive. Trace of water in the air leads to disproportionation to MnO2 and K2MnO4.
[Edited on 17-4-2019 by Bedlasky] |
Wait so how did you make a soln of MnO4 3-?
|
|
|
Wrecking Bereserker
Harmless

Posts: 25
Registered: 2-4-2019
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | Hypomanganates is very water sensitive. Trace of water in the air leads to disproportionation to MnO2 and K2MnO4.
[Edited on 17-4-2019 by Bedlasky] |
Exactly what happened to mine also i should say manganate is this way also when i saw the sample after a few days it has already went to MnO2 and
KMnO4
|
|
|
Bedlasky
International Hazard
    
Posts: 1227
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I made the solution as I described in first post. The problem with stability with solid hypomanganate was that it didn't in extremely alkaline
conditions. After reaction you haven't in mixture large amount of carbonate. The yield also dependent on temperature - if it's temperature too high,
hypomanganate decomposes.
In cold concentrated NaOH reduction (from my experiences) worked. How much is solution stable I don't know I didn't test it (but I plan it for the
future).
|
|
|
maxstar
Harmless

Posts: 1
Registered: 4-5-2019
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | Hello.
I made some solution of sodium hypomanganate cca 8 months ago. I am really interested in manganese chemistry and I prepared every oxidation state from
II-VI from KMnO4. But hypomanganate is really unstable and rare. This makes him really exotic compound.
I made him by reduction of KMnO4 by Na2S2O3 in 48% NaOH. All reagents was cooled in fridge before reaction because hypomanganates are more stable in
lower temperatures. In to the solution of 48% NaOH I added few drops of 25% solution of Na2S2O3.5H2O (Na2S2O3 isn't dissolve in water, but in 20%
NaOH). Then I added few drops of dilute aqueous solution of KMnO4. Colour of solution has turned in to green by presence of manganate. I returned
solution in to the fridge and after ten minutes solution turned colour in to turquoise by presence of hypomanganate. Solution was little bit cloudy,
because some NaOH in solution crystallized in the fridge before reaction.
On the internet I found that this compund have really interesting formula Na3MnO4.0,25NaOH.12H2O.
I plan to repeat this reaction in future and test how long solution stays turquoise and some redox chemistry with ethanol and acetone.
|
About a year ago I was busy with hypomanganates too. Made it in a similar way - a concentrated KOH solution (40%) mixed with some sodium sulfiet,
cooled it down in a fridge and started to drop potassium permanganate solution in it. I could observe the color change from pink violet to
cyan/turquise (hypomanganate) through green (manganate). Indeed it is not stable.
One way to stabilize it is with Barium. There are two pigments known from paintings - manganese blue and manganese green. The problem is - I could
find very controversial data about them. Some sources say that manganese blue is a mix of barium manganate with barium sulfate. Other sources say that
manganase blue is barium hypomanganate and manganese green is barium manganate...
There are several ways to obtain it - baking a mix of barium nitrate with manganese (IV) oxide (with sodium sulfate in some sources) or mixing
potassium permanganate with barium hydroxide (solutions).
I tried the last one and it worked, but very slow. It took weeks and finally I got a colorless solution (with a crust of probably barium carbonate on
the top as barium hydroxide was reacting with carbon dioxide from air) and a dark blue sediment. I also tried a different way - adding pure KOH to the
strong solution of potassium permanganate till it turned green and then adding barium hydroxide. Again, dark blue sediment appears. Just not sure if
it was manganate of hypomanganate. First I thought it was manganate, but later found those controversial facts about the colors of barium manganate
and hypomanganate...
[Edited on 5-5-2019 by maxstar]
|
|
|
| Pages:
1
2
3
4
5 |