| Pages:
1
2
3
..
5 |
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
sharing Awesome Chemical Pictures (Read first comment)
Hi everyone 
So i wanted to do a topic to share pictures of chemicals; interesting, expensive, rare, dangerous, synthetic, or what ever you want us to see.
I have the rather odd hobby of looking at new substances for quite a bit of time, and not to mention using it  i think i'm not the only one. So i wanted us to share pictures so we can enjoy looking at things we don't have access
to. i think i'm not the only one. So i wanted us to share pictures so we can enjoy looking at things we don't have access
to.
Here are the RULES:
1) Picture of a sample of compound
2) Attach a structure or formula or name o f the substance
3) If you like, a little explication of its uses
4) It must be a picture of a compound you own yourself. (woelen)
I'm starting !! Mercuric Cyanide Hg(CN)2
Double fun .. toxic mercury .. toxic cyanide ...uses ?? ... no idea realy :/

[Edited on 20-3-2019 by Chem Science]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
https://www.sciencemadness.org/whisper/viewthread.php?tid=26...
There's already a thread to post pretty pictures
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I would like to add an additional rule: It must be a picture of a compound you own yourself.
I add a picture of a saturated solution of Cl2 in CCl4. Its color is much closer to the color of liquid chlorine than normal saseous chlorine, which
is more green.

I made this by dissolving dried gaseous chlorine in pure CCl4, until no more gas could be dissolved. CCl4 dissolves quite some chlorine. I did not
measure it, but it is many times the volume of liquid. According to solubility info I found on internet, a saturated solution of Cl2 in CCl4 will
contain 7% to 8% by weight of chlorine. With a density of almost 1.6 grams per ml, this leads to roughly 0.11 to 0.12 grams of chlorine per ml of
liquid. Gaseous chlorine has a density of appr. 0.003 grams per ml, so this solution has 35 to 40 times more chlorine per unit of volume and hence has
a much stronger color.
I have no specific use for this sample. I use it as part of my element collection, displaying elemental chlorine at high concentration, without the
need to have liquid (and hence highly pressurized) chlorine around. I chose CCl4 as solvent. This cannot react with Cl2 anymore, the solution is
light-fast and stable on long-term storage.
[Edited on 20-3-19 by woelen]
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Yes but i want one of Reagents hehe (^.^)
Awesome sample woelen !! I edited the post and added your rule  that yellow is
beautiful that yellow is
beautiful
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
I definitely think these should be merged. Just also include ownership claim in the post where needed is enough.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
anything could be a reagent, but i get what you are saying
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Copper Citrate  To make ultra fine copper powder. how ever the copper powder
these gives is not pure, meaby these citrate is impure or the cooking i performed was bad. To make ultra fine copper powder. how ever the copper powder
these gives is not pure, meaby these citrate is impure or the cooking i performed was bad.

|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
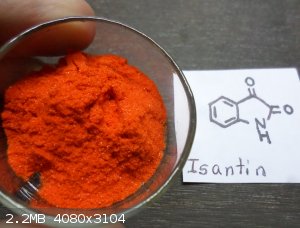
Isatin .. for synthesis 
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|

|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
A few more elements. I did not yet post them on my web page, they are fairly recent additions to my collection of elements:
A very pure sample of manganese, 99.99% purity, in an ampoule under Argon.

White phosphorus, little sticks, under water.

|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Harmine and harmaline hydrochloride crystals
Harmala alkaloids extracted from Peganum harmala (syrian rue) seeds, further purified by crystallization from brine.
These alkaloids are MAO inhibitors.
I further separate harmine and harmaline (DHH, dihydroharmine), the latter is further reduced to tetrahydroharmine (THH, leptaflorine) which is a weak
SSRI with interesting benefits for central nervous system (could promote neuroplasticity).

[Edited on 23-3-2019 by nimgoldman]
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Woow woelen That manganese seems awesome !!
i never heard of Harmine nimgoldman. very nice sample  Awesome to see !! Awesome to see !!

|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice naphthol! My organic class took the same compound and turned it into ethoxylnaphthalene earlier this semester- all was well and good until
someone (not a student) decided that the last remaining sample had to be taken out of the dessicator and returned to the oven (which had been turned
up to 80 oC). The student lost his sample to sublimation, but the lab smelled like lemon drops for days.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Hi DraconicAcid .. haha there's always someone that has to do it wrong xD But it's not bad to smell lemon 
Here are some Cobalt Complexes 
 
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Some Organic compounds  The Nitrophthalhydrazide was synthesized by me The Nitrophthalhydrazide was synthesized by me 
 
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Some Inorganic chemicals 
  
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
These two are very obvious when they are side by side like these 

|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
PETN and Gelignite when i was young and mad in science !
 
[Edited on 25-3-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
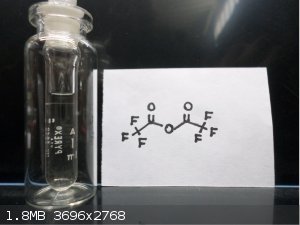
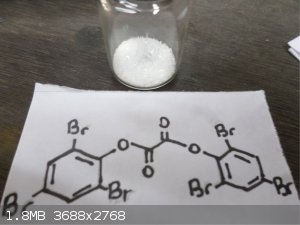
Wow nice pictures Waffles SS , first time looking at Pentaerythritol tetranitrate and Gelignite !! Here's some interesting stuff, Trifluoroacetic
anhydride and bis(2,4,6-Tribromo)Oxalate
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Trifluoroacetic anhydride. Something which still is on my wish list. I can buy it, but it is very expensive, so up to now I did not buy it.
This evening I made pictures of attractive samples, which I made myself. Many of the chemicals displayed here, I made myself. Some of them I
purchased.
The uploads are in multiple posts.
The first picture is a mix of Br2 and Cl2. I made this by filling a bottle of 250 ml with carefully dried Cl2 gas and then adding a small amount of
Br2. This Br2 then quickly reacts with Cl2 (it evaporates much faster than it normally does, because it reacts with Cl2, forming gaseous BrCl). The
resulting gas is a mix of BrCl and excess Cl2.
With a syringe I took 30 ml or so and blew this into an ampoule, which I sealed. This is the golden yellow gas in the big ampoule. To the remaining
gas, I added a few ml of CCl4 and swirled this around. The gas mix dissolves in the CCl4 and gives a bright red/orange solution. This bright
red/orange solution is a solution of BrCl together with excess Cl2 in CCl4. I also ampouled this solution.
This set of ampoules nicely shows that BrCl is orange/red in thick layers (or in higher concentration) and that it is golden yellow in thinner layers.
The liquid ampoule also shows the golden yellow color in the neck of the ampoule, where only a thin layer of liquid is viewed.

The two pictures below show the same ampoule, filled with K3Cr(C2O4)3.3H2O, but it shows how it looks like in fluorescent light and in tungsten light.
In fluorescent light it looks dark purple, in bright tungsten light it looks green. In daylight it looks grey.


|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Some more interesting compounds:
Mercury sulfide exists in two forms. A black form and a red form. The first sample contains lumps of HgS, which are pieces, containing both the black
form and the red form. These are from a natural source.

The HgS, shown in the picture below is synthetic, both samples are pure and nicely demonstrate that this compound exists in two forms. Both forms are
remarkably inert and do not dissolve in water, nor in strong acids.

Arsenic also forms interesting sulfides. There are two well-known sulfides, As2S3, knows as orpiment, and As4S4, known as realgar. Pure powdered As2S3
is golden yellow. Pure crystalline As2S3 is golden/yellow, with a greyish/brown tinge and a high polish. Such pure crystalline samples are rare and
expensive. More mundane sample usually are not shiny and have orange or red streaks in them, being As4S4 impurities. Sometimes, natural As2S3 also
contains white quartz, but that can easily be recognized, and that is easy to remove, because quartz is MUCH harder than As2S3. The picture below
shows three crystals of As2S3, containing small amounts of As4S4, which make the crystals appear somewhat orange instead of yellow.

The final two pictures in this post show very pure WO3 and WO3-x, with x appr. equal to 0.1, meaning that it is WO3, which is
slightly deficient in oxygen. It is well known that WO3 can be deficient in oxygen and if it is, this has a very strong impact on the color. Very pure
WO3 is pale yellow (color like sulphur, but slightly paler), but even a percent of oxygen deficiency leads to strong coloration in the blue part of
the spectrum. The color is very intense and hence the material is nearly black.


[Edited on 25-3-19 by woelen]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This is my recently acquired sample of cesium metal. It is large, a full 10 grams of metal.

This is some home-made PBr5, put in an ampoule. PBr5 is a very nasty chemical, which only can be stored indefinitely in a glass ampoule. It eats any
cap, even the PTFE-lined GL45 red caps. It heavily fumes in contact with air. I made it and quickly ampouled it, I did not do any further experiments
with it.

Below is a sample of P.A. grade FeSO4.7H2O. Normally, the material you buy is more like a dull grey/green, but the pure chemical has a very fine mint
green color. In air, it quickly loses its beautiful color, due to oxidation by oxygen in air. For this reason I ampouled some om my FeSO4.7H2O to keep
a rare and beautiful sample. For experimental purposes, one can better use (NH4)2SO4.FeSO4.12H2O, which has much better storage properties than
FeSO4.7H2O.

The final two pictures are ethylene diamine complexes of copper(II) and nickel(II). I made the copper(II) complex by dissolving as much as possible of
copper(II) oxide in 35% perchloric acid. I allowed the liquid to settle and decanted the blue solution of copper(II) perchlorate. To this I added a
30% solution of ethylene diamine in water, until. First you get a precipitate of copper(II) hydroxide, but on addition of more ethylene diamine, this
redissolves. I added two more drops of ethylene diamine solution and then put the solution aside and let it slowly evaporate, until I had a nice crop
of crystals. I did not let it evaporate to dryness. I decanted the liquid from the crystals, carefully dried the crystal mass in a coffee filter and
then let the mass dry in warm air. The dark purple crystals are the copper complex, Cu(en)2(ClO4)2.
The nickel complex was made by dissolving a soluble nickel salt in water and precipitating this with a solution of NaOH. This gives a precipitate of
Ni(OH)2, which on boiling for a while becomes coarser and easier to filter. I did not dry the Ni(OH)2, I simply rinsed it with water and then I
dissolved it in as little as possible of 35% HClO4. To this solution I added 30% solution of ethylene diamine in water. First you get a precipitate
again, next you get a dark blue liquid and finally you get a purple liquid. To this purple liquid I added a few more drops of ethylene diamine and
then I let this evaporate and worked up the crystals just as for the copper complex. Again, I did not evaporate to dryness.
Both complexes form nice crystals and can be obtained perfectly dry in the form of beautiful free flowing crystals. I ampouled both chemicals, because
they make very attractive display samples.


|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Woooww !! Awesome samples woelen   Nice Interhalogens !! I did not know about the WO(3-x), i have WO3 can i make WO(3-x) ? Nice Interhalogens !! I did not know about the WO(3-x), i have WO3 can i make WO(3-x) ?
That PCl5 is super nice !! i envy the fact that you make it your self !!
That Cesium ... yeah that's an Element <3 never actually seen in real life cesium  It's always nice to see pictures It's always nice to see pictures  It's my first time looking at
K3Cr(C2O4)3.3H2O, nice sample !! what is it used for ? It's my first time looking at
K3Cr(C2O4)3.3H2O, nice sample !! what is it used for ?
Okay .. it's difficult to keep up, let's see if you like these next samples 
So First is Hematoporphyrin Dihydrochloride al 75% Then we have the Precursors and actual 2,4.Dinitrophenylhydrazine and last is 1,5-Diphenylcarbazide
   
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Copper nitrate form a silver refining process, left the beaker aside an this single beauty grew.
Sadly I do not have better pictures as I gave it away as a present a year ago.


|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by DrScrabs  | Copper nitrate form a silver refining process, left the beaker aside an this single beauty grew.
Sadly I do not have better pictures as I gave it away as a present a year ago.
|
That looks like sulphate instead
|
|
|
| Pages:
1
2
3
..
5 |