| Pages:
1
2 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well then, use ammonium acetate, acetic acid, H2O2 and copper, or bubble with air and leave out the H2O2. THat should work too.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by guy
I was hoping that this reaction would be good yielding since it is very similar to the extraction of gold process.
4Au + 8CN<sup>-</sup> + O2 + 4H2O ---> 4[Au(CN)2]<sup>-</sup> + 4OH<sup>-</sup>
|
Ah, but in this reaction involves gold complexing with the cyanide anion, which removes gold from the left side of the equation. This pushes the
equilbrium to the right, so that gold (solid) continually gets "dissolved".
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by Darkblade48
Ah, but in this reaction involves gold complexing with the cyanide anion, which removes gold from the left side of the equation. This pushes the
equilibrium to the right, so that gold (solid) continually gets "dissolved". |
Yes, so does this reaction, as copper complexes with ammonia.
2Cu + O2 + 2H2O + 8NH3 <------> 2[Cu(NH3)4]<sup>2+</sup> + 4OH<sup>-</sup>
*************************************
Okay, this reaction would go a lot faster some ammonia to start with. Eventually this reaction should start becoming more and more basic and start to
form more ammonia on its own.
I tried to do some math to calculate the amount of NH3 needed.
Cu(OH)2 <----> Cu2+ + 2OH- K1=1.6 x 10^-19
Cu2+ + 4NH3 <------> [Cu(NH3)4]2+ K2=1.2 x 10^12
K1 x K2 = 1.92 x 10^-7
Say I want to make 2 moles of [Cu(NH3)4]2+ in 1L water.
[2][2]<sup>2</sup>
------------ = 1.92 x 10^-7
[NH3]<sup>4</sup>
[NH3] = 80.3 M 
80.3 + 4(2) = 88.3 M needed.
Therefore I need 5.83kg of (NH4)2SO4. IS THIS RIGHT?? IS THIS RIGHT??
******
The efficiency of this reaction would be greatly increased if the Ksp of Cu(OH)2 is increased. I have tried searching for solubility graphs of
Cu(OH)2 but got nothing. This information would be very useful. If the solubility can be increased, less (NH4)2SO4 will need to be used, and less
contamination.
[Edited on 12/14/2005 by guy]
[Edited on 12/14/2005 by guy]
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
<b>Using Activated carbon</b>
This site talks about the use of Carbon in Pulp (CIP) in the process of gold extraction. Could this be applicable for my tetraaminecopper sulfate
process also?
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
No, wrong calculations!!
I forgot to add the K of NH4+ + OH- --> NH3. Now the Equilibrium constant is 3.703 x 10^3. Very large, so I will be getting some good yield!  I'll post back if I ever get around to doing this. I'll post back if I ever get around to doing this.
The final reaction:
2NH3 + (NH4)2SO4 + Cu(OH)2 -------> [Cu(NH3)4]SO4 + H2O
{[Cu(NH3)4]<sup>2+</sup>}
-------------------------------------- = 3.703 x 10<sup>3</sup>
[NH3]<sup>2</sup>[NH4<sup>+</sup>]<sup>2</sup>
Does anyone have any ideas on how to design this thing? I'm thinking a bucket with the solution (ammonia added in small intervals) and copper
(elevated a few inches) and airstones connected to an airpump.
[Edited on 12/16/2005 by guy]
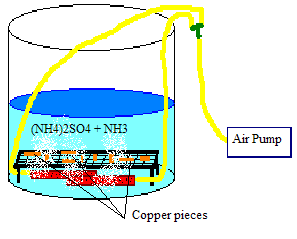
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Yes! I finally did it and I made a whole bunch of Cu(NH3)4SO4, which can be heated to drive off the ammonia. Should I post the whole procedure in the
Prepublication forum? First I'm gonna to take some pictures.
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by guy
Yes! I finally did it and I made a whole bunch of Cu(NH3)4SO4, which can be heated to drive off the ammonia. Should I post the whole procedure in the
Prepublication forum? First I'm gonna to take some pictures.
|
I was under the impression that heating the complex salt would force the ammonia out, thereby destroying your product.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Why would it force the ammonia out? It would first form tetraamine copper hydroxide which reacts with the Ammonium sulfate to form ammonia and water
and tetraamine copper sulfate.
[Edited on 12/21/2005 by guy]

|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I guess I should have worded it a little more carefully. I'm under the impression that the ammonia would just evaporate off, and might do so more
readily under heat. The sample of the complex salt that I have slowly seems to be losing its dark blue colour, and everytime I open the jar, the odor
of ammonia escapes from the bottle.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Hey, guy, how many days did you take to dissolve your copper?
In the same vein, would a similar reaction involving the chloride complex of copper ion work?
Cu + 4Cl- + O2 + 2H20------------>[Cu(Cl)4]2- + 4OH-
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
No, with chloride it does not work. You instead get insoluble basic copper chloride, which in fact is copper hydroxide/chloride, something like
Cu(OH)Cl.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
It took me three days to dissolve all the copper. I think it took a little longer than it should have because for one day I didn't put the copper
directly over the air bubbler. Chloride might not work well becuase if the formation constant for [CuCl4]2- is much smaller than for forming
tetraaminecuprate. And anyways, ammonia and ammonium sulfate are way cheaper than salt. And It wont have any contaminiants in the end once you heat
it and drive off the ammonia.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
VERY neat!
I wouldn't have thought it'd work that well! So you didnt use any H2SO4 to acidify?
So let me get this straight, all you had in there was ammonium sulphate and ammonia. How did you ensure that the ammonia didnt evaporate, because you
must have put many cubic liters of air through there! Did you refill with ammonia now and then? The stink must have been enormous!
I can't see an easy way to trap the ammonia either... so you can't do that inside I suppose.
Still, very nice.
Let us know how easy it is to decompose the tetraamine sulphhate to normal copper sulphate.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Well, to trap the ammonia i had to design it the pump would have to stay inside the container so it would keep recirculating the air and ammonia.
I'll post the pic.
I did not use any acid since I have none.  HAHA. Anyways maybe I could use this
copper sulfate somehow to make sulfuric acid. HAHA. Anyways maybe I could use this
copper sulfate somehow to make sulfuric acid.
[Edited on 12/21/2005 by guy]

|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Heres the inside

|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
NO! What happened?!
When I evaporated the solution, copper HYDROXIDE precipitated out. How is this possible? Maybe I measured my reactants wrong. Does ammonium sulfate
(from fertillizer) have hydrates?
These are my reagents.
10g Cu
54.5 g NH3 (from 10% solution) so 5.45 g of NH3
20.78 g (NH4)2SO4
Could this be a possible explanation?
2Cu + O2 + 4NH3 + 4NH4(+) <-----> 2[Cu(NH3)4](2+) + 2H2O
As I heat the solution in an attempt to collect the product, the remaing ammonia in the solution is first driven off. This shifts the equilibrium
back left, forming once again copper hydroxide.  It seems like the only way to
separate is to precipitate it using 100% ethanol or isopropyl. There is a lot of water so should I first try and remove some of the water with
anhydrous CaCl2? Man, Im soo close yet so far away It seems like the only way to
separate is to precipitate it using 100% ethanol or isopropyl. There is a lot of water so should I first try and remove some of the water with
anhydrous CaCl2? Man, Im soo close yet so far away 
[Edited on 12/22/2005 by guy]
[Edited on 12/22/2005 by guy]
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I figured your product would decompose, that's why I suggested against heating it (the decomposition temperature of the complex is supposedly 120 C.
You can use methanol as well to precipitate out the salt (at least that's what I used, it's cheaper for me to get MeOH rather than IPrOH and EtOH in
high purities).
Depending on how much water you have, you may or may not find that trying to get rid of the water with anhydrous CaCl2 is effective. From the looks of
it though, you might have to use a lot of CaCl2 to suck up all that water.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
[Cu(NH3)4](2+) + 2OH(-) <---> Cu(OH)2 + NH3 (g)
Maybe the above reaction is favored when the temperature is higher. Reminds me of the temperature senstive cobalt chloride complex. There has to be
some hydroxide ions inside the solution leftover. Unless the reagents are used in stoichiometric amounts and the reaction goes to completion where
everything is used up, the solution is not going to be neutral. Perhaps you should test the solution to see if its neutral?
Else, you might have to filter out the copper(II) hydroxide, add ammonia to redissolve it, and add magnesium sulphate to swap the ions. This is under
the assumption that you do not have sulphuric acid right?
Related website worth taking a look:Etching with air regenerated Cupric chloride
[Edited on 22-12-2005 by darkflame89]
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Arg! Its impossible to decompose the tetraamine copper sulfate. I washed completley dry with denatured alcohol. Then I heated it and it still turned
to copper hydroxide. Why??  
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Hmm, I feared this might happen - it is because you essentially eliminate ammonium sulphate, and its decomposition products.
How do you know it's copper hydroxide btw? It decomposes actually under heat to form black CuO. Is that what you get?
Or are you getting just dehydrated CuSO4, which is white, and dissolves in H2O to form the blue CuSO4 solution?
PS you heat it at what temperature? Please be more specific! Weigh it before and after heating! That should tell you what you got.
[Edited on 28-12-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
YAY!
Thanks for chemoleo for pointing out if it turns to CuO. It didnt turn to CuO. This time I heated it much longer and it turned to a white solid
which is dehydrated CuSO4!! It figured out why before it turned to copper
hydroxide when I put it in water. I didnt heat it completly so some of the remaining tetraamine sulfate turned to copper hydroxide when I added it in
excess water. It figured out why before it turned to copper
hydroxide when I put it in water. I didnt heat it completly so some of the remaining tetraamine sulfate turned to copper hydroxide when I added it in
excess water.
So I guess this way works for making CuSO4.  
How long does it take for the anhydrous copper sulfate to dissolve? It looks like its taking a while.
[Edited on 12/28/2005 by guy]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Hehe that's good to hear.
Yes, I recently played with analytical anhydrous CuSO4, and I was quite surprised how long it takes to dissolve.
Please, do something for me.
Take your dried, alcohol precipitated tetraamine copper sulphate. Weigh whatever quantity you like.
Then heat it, until it almost starts to glow, and all colour has gone. Then WEIGH it again please.
You can do it even in that very same crucible as long as you determine the weight of the crucible.
Furthermore - how do you know it's CuSO4? Does EVERYTHING dissolve, or is there a residue, of whatever nature? Does the product dissolve ENTIRELY in
H2O, with no remainder?
That's what I want to know!!!! It's good stuff, seriously, but you have to learn to describe what you did!
[Edited on 28-12-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
So far I have heated it up until it is a white solid. First it was 20g of tetraamine copper sulfate (The measurements are not accurate since I only
have a kitchen scale). Then after heating to a white solid, the mass is approximately 10g.
I have to wait 'til tommorow to see if the product dissolves entirely. There seems to be some CuO residue in the product.
[Edited on 12/28/2005 by guy]
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Final Product
After dissolving the anhydrous salt and recrystallizing it, I finally obtained the copper sulfate pentahydrate crystals.

|
|
|
| Pages:
1
2 |