wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Blue tungstic or molybdic acid ?
I was hoping to make tungstic acid or possibly molybdic acid from the small metal parts removed from the filament assemble of a dismantled magnetron,
see first pic.
I first cleaned parts in 28% HCl for an hour with shaking to oxygenate the acid, then rinsed in water. There were some small pieces of filament left
on the parts that had a grey colour compared to the black colour of most of the parts. The filament may not be pure tungsten which could account for
the different colour or the parts may be molybdenum. I am assuming tungsten or molybdenum from the source and the facts that they are not magnetic and
do not dissolve hot oxygenated HCl.
I put the parts about 400mg in 5mL of 12% H2O2 in a test tube. There was some slight bubbling which gradually increased over 20 minutes to vigorous
bubbling so I place the test tube in a beaker of cool water to reduce the chances of thermal runaway. After 5 hour the bubbling had almost stopped and
the solution was a clear light straw colour. When I swirled the solution a transient (few seconds) blue colour appeared a round the parts.
When I checked the test tube 12 hours later the solution is now a very intense blue. It looks black unless viewed threw the meniscus. See the second
pic, sorry its so out of focus my camera’s focus does not work very well but it does show the colour intensity and its hue.
I was expecting a straw coloured solution of either tungsten acid or molybdic acid.
Can anyone suggest what the blue colour is?
I was planning on testing for molybdenum by the very unusual ammonium phosphomolybdate salt (NH4)3PMo12O40


|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
1. Could you possibly take a better second picture?
2. Perhaps there is some copper in your source that is forming the very dark blue colour. That being said, it doesn't look like a copper salt... it
seems a bit too dark/green
In my limited experience with molybdic acid (Which is extended and limited to me getting a free container of some at work once) it is much more white
(atleast as a solid) than straw coloured, although a couple sources I can see label it as straw. I'll be out in my lab this aft, i'll check that for
you.
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/Molybdenum_blue
|
|
|
Foeskes
Hazard to Others
  
Posts: 156
Registered: 25-2-2017
Member Is Offline
Mood: No Mood
|
|
I made tungstic acid once from bulk tungsten using a molten mix of NaNO3 and NaOH.
It is really violent and after it gets hot enough the reaction is self sustaining. The heat generated heated the crucible red hot.
I recomend nickel crucibles though I used stainless and got Cr(VI) contamination so I used some reducing agent to reduce that. I then used HCl to form
Tungstic acid and filter.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
we used to get this stuff all the time at my old work(manufacturing plant for tungsten carbide mining tips) when someone mixed an incorrect ratio of
wax and the tips crumble in the sinter furnace.
It can be made 'intentionally' by mixing Powdered W metal and WO3 and firing in a furnace.
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Maybe a mixed tungsten oxide... Is it an actual solution or a suspension?
Batoussai.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Traces of heteropolyacids formed from tungsten/molybdenum and small amounts of phosphorus, silicon, or arsenic form extremely dark blue colors when
partially reduced. This is the basis for a number of analytical chemistry techniques for quantifying phosphate, arsenic, or even reducing species like
proteins or phenols. For an example, see Folin-Ciocalteu's reagent.
You are probably looking at a very small amount of colored species.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Thanks for the suggestions everyone.
After ten hours there is no clear liquid at the top of the test tube. I assume the blue is in solution. The colour is not taken up by ethyl acetate
but is with MEK see pic.
When I add H2O2 to the blue solution it disappears in a few seconds to be replaced by a slight yellow colour. It is also destroyed when exposed to
air in minutes (drops on a white plate). When dry the yellow solution leaves a distinct yellow residue.
I have tried to dissolve what the seller claimed was molybdenum wire but after fours hours in H2O2 12% apart from slight bubbling no reaction and the
wire remains shiny. I assume the wire is not molybdenum.
Apparently molybdenum copper alloys (actually a mixture) are common but as there was no reaction to oxygenated HCl I assume the metal did not contain
copper.
Tungstic acid is yellow but is insoluble in water. Molybdic acid is yellow and is soluble in water and is reduced to blue molybdenum by molybdenum
metal and blue molybdenum is readily oxidised back to Molybdic acid. Note: there are molybdenum parts remaining in the test tube of the blue solution.
I conclude from the above the blue compound is molybdenum blue. I wanted Tungstic acid but the blue M is interesting and very unexpected.
It could be the P hetro acid as phosphoric acid is commonly used to stabilise H202 but I doubt there was sufficient phosphoric acid to produce such an
intense colour and I think the blue hetro acid is not soluble. When testing for P phtometricaly I don't know how they avoid the blue molybdenum (homo
acid) interference ???

Blue M in 2 ml of MEK extracted from about 0.2ml of blue solution. Still a strong blue colour unless viewed back light from the torch. Its the same
hue as the original water solution.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Both tungsten and molybdenum form blue compounds in oxidation state +5. I think that the blue compound is formed by the reduction of molybdenum(VI) or
tungsten(VI) by other metals in the parts you intended to dissolve.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I managed to caputure the blue m acid forming slowly on video.
Its speeded up 500 times,one frame every ten seconds.
Attachment: mblue.avi (7.5MB)
This file has been downloaded 762 times

The blue M was first oxidised with H2O2 until the blue colour was a straw colour (believed to be Molybdic acid) The metal part believed to be
molybdenum then slowly reduces the Molybdic acid to a blue molybdium acid containing molybdium in a lower oxidation state. Presumably the molybdenum
metal is oxidised and passes into solution. An orther interpretation is the metal is an alloy of molybdenum and it’s the orther metal/s that reduce
molybdic acid.
[Edited on 7-5-2018 by wg48]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
In every magnetron I have taken apart there has been a coil of a brittle wire around the center wire which I am led to believe is thoriated tungsten.
Have you removed this coil before taking the photos or did your magnetron not have it? I have a few magnetron carcasses I would like to play with but
I do not want to make aqueous thorium by accident. So, in your reading, the center filament has no thorium in it?
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Plunkett  | | In every magnetron I have taken apart there has been a coil of a brittle wire around the center wire which I am led to believe is thoriated tungsten.
Have you removed this coil before taking the photos or did your magnetron not have it? I have a few magnetron carcasses I would like to play with but
I do not want to make aqueous thorium by accident. So, in your reading, the center filament has no thorium in it? |
Usually during the dismantling process the tungsten filament breaks, as you suggest the filament is usually very brittle due to its high temperature
in operation in a used magnetron. The metal part I used had most of the filament removed except for small pieces near the attachment points, which
could have been removed leaving perhaps only a millimetre.
Yes the filament may be thoriated tungsten (1 to 2%) and that is a minor concern but as in all experiments don’t let any chemical contaminate you or
your environment other than that I don’t think you should be concerned.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
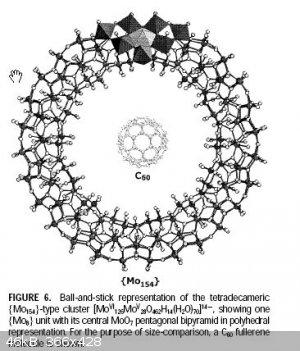
I found a note on molybdium blue; Attachment: mblue.pdf (737kB)
This file has been downloaded 521 times It's an interesting read.
Wow, there are many different M blues types. A typical building block is a pinwheel type of structure shown below. Apparently molybdium metal can
reduce molybdiumates to a blue so I assume it can reduce the acid too as confirmed by my experiment. There are several synthesise using various
reagents but H2O2 and molybdium metal is hard to beat for its simplicity
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
My pure (allegedly) molybdenum pieces arrived yesterday so I tried to make some blue Mo. The pic shows two test tube containing small pieces of Mo in
about 1ml of 12% H2O2. The one on the right also contains one drop of 33% sulphuric acid to help stop the decomposition of the H2O2. It initially did
not heat up and was less intensely coloured after one hour compared to the plain H2O2 on the left. Now after 12 hours they are very similar. No blue
as yet and both contain unreacted Mo now black.

[Edited on 24-5-2018 by wg48]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I became impatient waiting for the H2O2 to beused up in the reaction to allow the blue Mo to be created so I heat both test tubes in a water bath to
60C to increase the reaction rate with the Mo metal and or decompose the H2O2.
After an hour the test tube had contained plain H2O2 was much lighter colour and when gentle agitated developed the blue colour at the bottom of the
test tube then faded back to a light straw colour. The test tube that also contained a drop of sulphuric acid had not changed colour and on agitating
only developed a brief and slight blue colour.
Assuming the Mo is pure it appears the molybdenum metal reduces straw-coloured Mo oxide to the blue Mo. This does not exclude impurities in the H2O2
being responsible for the blue colour.
The pic below shows the two test tubes in the water bath. The left one is the plain H2O2. Just visible are the wisps of blue Mo. I expect it will turn
all blue when the H2O2 is used up. Its a moly clock reaction that takes many hours.

[Edited on 24-5-2018 by wg48]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by wg48  | I became impatient waiting for the H2O2 to beused up in the reaction to allow the blue Mo to be created so I heat both test tubes in a water bath to
60C to increase the reaction rate with the Mo metal and or decompose the H2O2.
After an hour the test tube had contained plain H2O2 was much lighter colour and when gentle agitated developed the blue colour at the bottom of the
test tube then faded back to a light straw colour. The test tube that also contained a drop of sulphuric acid had not changed colour and on agitating
only developed a brief and slight blue colour.
Assuming the Mo is pure it appears the molybdenum metal reduces straw-coloured Mo oxide to the blue Mo. This does not exclude impurities in the H2O2
being responsible for the blue colour.
The pic below shows the two test tubes in the water bath. The left one is the plain H2O2. Just visible are the wisps of blue Mo. I expect it will turn
all blue when the H2O2 is used up. Its a moly clock reaction that takes many hours.
[Edited on 24-5-2018 by wg48] |
The brown colour in right tube looks like Br2 or NO2 gas TBH
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fusso  | Quote: Originally posted by wg48  | I became impatient waiting for the H2O2 to beused up in the reaction to allow the blue Mo to be created so I heat both test tubes in a water bath to
60C to increase the reaction rate with the Mo metal and or decompose the H2O2.
After an hour the test tube had contained plain H2O2 was much lighter colour and when gentle agitated developed the blue colour at the bottom of the
test tube then faded back to a light straw colour. The test tube that also contained a drop of sulphuric acid had not changed colour and on agitating
only developed a brief and slight blue colour.
Assuming the Mo is pure it appears the molybdenum metal reduces straw-coloured Mo oxide to the blue Mo. This does not exclude impurities in the H2O2
being responsible for the blue colour.
The pic below shows the two test tubes in the water bath. The left one is the plain H2O2. Just visible are the wisps of blue Mo. I expect it will turn
all blue when the H2O2 is used up. Its a moly clock reaction that takes many hours.
[Edited on 24-5-2018 by wg48] |
The brown colour in right tube looks like Br2 or NO2 gas TBH
|
That colour of the right test tube is too dark caused I suspect by the auto white balance on the cam and or the LED light. For better colour I can fix
the colour balance while viewing a plain white card. To my eyes the colour is the same as the first pic.
Below is an other pic, still not the correct colour. Note there is more wisps of blue in the left tube. It will soon be all blue

|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The brown/orange compound is a peroxo complex of H2O2 and molybdate. I have some Na2MoO4 and when I dissolve this in water, add some acid and then add
some H2O2 I get a brown liquid. When it becomes more dilute it becomes orange/brown.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | | The brown/orange compound is a peroxo complex of H2O2 and molybdate. I have some Na2MoO4 and when I dissolve this in water, add some acid and then add
some H2O2 I get a brown liquid. When it becomes more dilute it becomes orange/brown. |
The orange colour being caused by a peroxide complex would explain why the orange develops and then fades as the Mo metal reduces the peroxide. In the
last pic I posted the test tube on the left is almost colourless because the peroxide has been used up and the Mo metal is now starting reduce the
molybdate to the blue Mo. Presumable if I add sufficient peroxide to the blue Mo it will first turn almost colourless than with sufficient peroxide
to form the complex it will turn orange again. I will try that.
Some more observations:
The blue Mo at least at first is a precipitate or colloid as it does settle slowly leaving a clear solution, but a few hours later it fills the whole
solution and then does not settle. (I need to double check that)
The original test tube of blue Mo is now very intensely coloured with some slight white deposits at the bottom of the test tube and a blue ring
deposit at the meniscus
The two recent test tubes have been incubated at 60C for several hours both had turned blue but now the test tube that had a drop of sulphuric acid
has formed a white gel above a few millimetres of a blue solution. The plain peroxide test tube also has some white precipitate in it.
Below is pic of the test tubes. The one contained the drop of acid is on the left. I do not know if there is any Mo metal left in them.

|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
A form of molybdenum blue was prepared according to the procedure in "Thomas' Chemistry" "Synthesis of Molybdenum blue"
https://thosci.com/synthesis-of-molybdenum-blue/
The product is a very dark blue color when in solid form. In fact it is difficult to capture the blue with my phone camera, it looks black. See photos
below. Diluted solutions shows the blue better, but I did not take a photo.
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
wg48: If you add excess of H2O2, there shouldn't be any molybdenum blue - because Mo and other metals are oxidized in to some stable higher oxidation
state, so there isn't any reducing agent. If you want to make some solid molybdate than you add some ammonia or alkali metal hydroxide and boil it for
a while. If you use only 12% H2O2 there shouldn't be any runaway. If solution will start bubble too much, add small amount of water. Mo-peroxide
complexes are unstable in alkaline media and they decompose. In acidic media they are quite stable.
Probably best option is making ammonium heptamolybdate, because it's less soluble than Na2MoO4. If you have nitric acid you can make some MoO3 by
adding huge excess of HNO3.
|
|
|
wg48temp9
National Hazard
   
Posts: 787
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | wg48: If you add excess of H2O2, there shouldn't be any molybdenum blue - because Mo and other metals are oxidized in to some stable higher oxidation
state, so there isn't any reducing agent. If you want to make some solid molybdate than you add some ammonia or alkali metal hydroxide and boil it for
a while. If you use only 12% H2O2 there shouldn't be any runaway. If solution will start bubble too much, add small amount of water. Mo-peroxide
complexes are unstable in alkaline media and they decompose. In acidic media they are quite stable.
Probably best option is making ammonium heptamolybdate, because it's less soluble than Na2MoO4. If you have nitric acid you can make some MoO3 by
adding huge excess of HNO3. |
The Mo was in excess. It was only test to check the metal I had received really was Mo.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|