chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
"Perchloro" organics
What is known about, and how are called, compounds with structural fragment O3Cl-C?
In analogy with compounds called "nitro" compounds, with structural fragment O2N-C, the fragment O3Cl-C should be called
"perchloro".
But "perchloro" is also used for organic compounds that lack C-H bonds, having only C-Cl bonds, but no Cl-O bonds.
So what is specific term for O3Cl-C?
|
|
|
Thulium
Harmless

Posts: 8
Registered: 25-3-2018
Location: Socialist Soviet of Californiastan
Member Is Offline
Mood: Electropositive
|
|
I suspect this may be a case of "if you can isolate it, you can name it". A very quick scan of the literature on the internets shows no references to
any of these compounds. This isn't terribly surprising to me since perchlorate esters are already incredibly unstable (http://www.sciencemadness.org/talk/viewthread.php?tid=1081) and the perchlorate anions stability is largely due to the closed shell provided by
the four highly electronegative oxygens. Replacing an oxygen with a carbon would eliminate this last stabilizing force. As for why perchlorocarbons
are called such, I suspect it's to be analogous with perfluorocarbons in which case there is no possibility of confusion with "perfluoric acid".
I would love it if you, or anyone, can prove me (and whoever named the "perfluorocarbons") wrong on this by synthesizing what I would suspect to be s
very energetic material indeed 
|
|
|
Texium
|
Thread Moved
26-3-2018 at 11:32 |
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
If you have access to a cyclotron you could make perhaps it from perchloramide.
N13H2ClO3 ---> C13H2ClO3• + ve
Before you try it though, i have only read of Perchloramides being made from secondary amines, the electron donation from them might help with
stability too. Note that it would be a radical.
Playing around a bit with resonance structures this is the most stable looking thing i can find. As for a name, they are similar in structure to
Nitronic acids so i would suggest Perchloronic acids if they exist, which they probably do not, but here is a picture anyway:
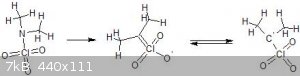
[Edited on 26-3-2018 by Σldritch]
|
|
|
Thulium
Harmless

Posts: 8
Registered: 25-3-2018
Location: Socialist Soviet of Californiastan
Member Is Offline
Mood: Electropositive
|
|
This question sort of bugged me all day today, and I was thinking maybe something similar to a Wurtz reaction could be done with Perchloryl Fluoride
(which is amazingly stable for such a cluster of electronegativity). Looking at the wikipedia page for Perchloryl Fluoride (https://en.wikipedia.org/wiki/Perchloryl_fluoride), it seems there are two references to what they call "alkyl perchlorates" (somewhat ambiguous
term with perchlorate esters) being formed in a Friedel-Krafts type reaction with Perchloryl Fluoride and hydrocarbons, featuring the C-ClO3 bond the
OP described. I unfortunately do not have access to either of the books cited though to verify that they do in fact mention these compounds being
characterized.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
You mean perchloryl compounds? https://en.wikipedia.org/wiki/Perchlorylbenzene
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Thulium  | | This question sort of bugged me all day today, and I was thinking maybe something similar to a Wurtz reaction could be done with Perchloryl Fluoride
(which is amazingly stable for such a cluster of electronegativity). Looking at the wikipedia page for Perchloryl Fluoride (https://en.wikipedia.org/wiki/Perchloryl_fluoride), it seems there are two references to what they call "alkyl perchlorates" (somewhat ambiguous
term with perchlorate esters) being formed in a Friedel-Krafts type reaction with Perchloryl Fluoride and hydrocarbons, featuring the C-ClO3 bond the
OP described. |
Alkyl or aryl?
The original works with perchloryl fluoride and aluminum chloride (sic!) were late 1950s. 60 years ago.
If what you want is electrophilic perchloryl cation then you have other fluoride acceptors. For nitration, a popular reagent is nitryl
tetrafluoroborate, NO2BF4.
So what can be used to make ClO3F more electrophilic? BF3??PF5?AsF5?SbF5?
|
|
|
Thulium
Harmless

Posts: 8
Registered: 25-3-2018
Location: Socialist Soviet of Californiastan
Member Is Offline
Mood: Electropositive
|
|
Quote: Originally posted by chornedsnorkack  |
Alkyl or aryl?
The original works with perchloryl fluoride and aluminum chloride (sic!) were late 1950s. 60 years ago.
If what you want is electrophilic perchloryl cation then you have other fluoride acceptors. For nitration, a popular reagent is nitryl
tetrafluoroborate, NO2BF4.
So what can be used to make ClO3F more electrophilic? BF3??PF5?AsF5?SbF5?
|
First a thanks to DraconicAcid for clarifying these compounds do exist and are called Perchloryls, and presumably aryl if we are talking about
perchlorylbenzene.
This is new territory for me, but for there synthesis, it looks like it can be done in water with AlCl3 acting as the fluoride acceptor, producing HCl
in the process.
Now I'm now if anyone's ever synthesized Perchloryl Methane... The fuel to oxidizer ratio looks... energetic 
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Turns out BF3 can be used for perchlorylation of aromatics but is less active than AlCl3. Reported by Olah, 2003 - source is from 1966, JACS 88, 3819.
Inmann et al.
|
|
|