weird_alice
Harmless

Posts: 1
Registered: 21-10-2017
Member Is Offline
Mood: No Mood
|
|
Why is infrared light a heating light?
Used for that or called that way.
Why is not ultraviolet, isn't it of higher energy?
If UV is not heating, then where does the energy go? Reflected to somewhere? Passes trough something?
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
I think that infrared light is associated with heating mainly because it is relatively easy to produce a lot of infrared energy with common materials,
to produce ultraviolet needs temperatures well above the melting points of most engineering materials,
or more usually emission from exited gasses, usually mercury for uv.
A many kW uv lamp is very much more expensive than electrically heated wires etc.
and, a uv lamp of several kW would blind people really quickly, create lots of toxic reactive ozone, kill all cellular life .......
but it could be used as a heater.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Current LEDs however can produce UV light as well. There are even dedicated low power 'blackleds' i.e. a blacklight LED in a low power torch. But they
produce barely heat.
In stars, the hotter the star (spectral class O, B or A), the more energy it delivers per kilogram of material.
If our Sun were 10000K (more blue light and less infrared) instead of 5800K, the Earth would be scorched away and life would not be possible.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
At least part of the distinction might be because IR heats things, but doesn't generally cause other changes.
Visible and UV light produce photochemical changes (in some materials) which are different from those caused by simple heating.
|
|
|
barbs09
Hazard to Others
  
Posts: 113
Registered: 22-1-2009
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Hi, I will probably be corrected, but different things adsorb different wave lengths differently. We being mainly water adsorb the IR spectrum
efficiently. Whereas longer wave lengths such as radio waves or shorter wave lengths such as X-rays/ gamma rays and not readily adsorbed by our tissue
and we are effectively rendered transparent to them.
Obviously our cells are not completely transparent to very short wave radiation, otherwise it would pose no risk.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
I agree with the above answers. It is very difficult to produce large quantities of blue, indigo, violet, UV light if you are going to do it by
heating a material. You get that blackbody style graph. Most of the emission is in the IR.
You could buy a UV laser.
Example:
http://www.cnilaser.com/UV-Laser-257nm.htm
This is 1 to 15 mW which is nothing.
A violet blue laser
http://www.cnilaser.com/blue_laser400.htm
It is 300 mW.
That should be enough to burn holes in some black PVC tape.
https://www.amazing1.com/products/355nm-ultraviolet-laser-mp...
355nm Ultraviolet Laser MPL-W-355 Solid State 100-800mw Pulsed
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
All light is 'heating', if it gets absorbed. In fact I'd broaden that to say all electromagnetic radiation is 'heating' if it get absorbed. I had a
21W blue LED glue cure lamp (450nm), and I could definitely feel that on my hand.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
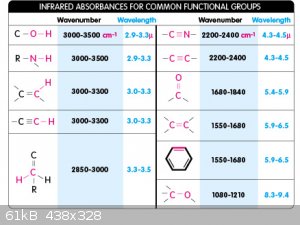
All electromagnetic radiation heats matter up, how efficiently is to do with how well the material absorbs particular wavelengths - laser pointers
above ~100mW can easily melt and/or set fire to dark objects in the visible wavelengths with a focusing mechanism. Microwaves heat food up because
they're absorbed very well by the OH bond in water, causing water molecules to vibrate by oscillating the bonds. Here's an absorption chart of peak
wavelength absorption of certain functional groups (these are normally used for IR spectrometry), this should give you some insight into why organic
compounds can be heated in this manner.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|