bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Solvents purification by drying then freezing them!
Hi guys!
I don't have any idea about how can this be useful but I just would like to discuss it with you
We all know that we can purify solvents like acetone & ethanol using drying agents then distillation but what about using drying agents then
freezing it?
these two solvents have very low melting points which I believe makes separating them from water relatively easy, may be easier then distillation!
Theorically there are at least a few chemicals can be used like CaO, I'm not sure though..
does it really work well???
[Edited on 30-4-2017 by bluamine]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Freezing was how acetic acid was purified for a long time. That's why it's called "glacial".
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Interesting information, I never looked out to know the reason why it was called glacial before!
Anyway, acetic acid seperation is different because it doesn't form an azeotrope with water, but alcohol does (95%)
https://en.wikipedia.org/wiki/Azeotrope_tables
[Edited on 30-4-2017 by bluamine]
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
as far as I know an azeotrope has nothing to do with freezing behavior.
It works in many places where destillation failes. (m-,p- xylene separation)
Maybe there is an antifreeze effect and the solvent and the water will freeze together.
But you are talking about drying agents anyway so what about adding the agent and filtering?
I mostly use cacl2,mgso4 or molecular sieves 3A and then do a filtration through cotton.
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by subskune  | as far as I know an azeotrope has nothing to do with freezing behavior.
It works in many places where destillation failes. (m-,p- xylene separation)
Maybe there is an antifreeze effect and the solvent and the water will freeze together. |
I don't know how do azeotropes act when they are cooled to the freezing points of their composants
Magnesium sulphate is slightly soluble in alcohol & most of it can be filtered out easily, but I guess calcium chloride may form some complexes
with alcohol, that's why I never used it for this purpose
[Edited on 30-4-2017 by bluamine]
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
"I don't know how do azeotropes act when they are cooled to the freezing points of their composants"
Azeotrope is defined as a mixture that cannot be separated or altered by distillation. The components will vaporize and condense with exactly the same
proportion and are thus unchanged. Azeotropes don't apply when the mixture is being frozen.
The problem is that mixtures of miscible liquids will have melting and boiling points that are an intermediate of the components. For example methanol
(bp ~60C) and water (bp 100C) are miscible with each other. A 50/50 mixture of methanol and water will not boil at 60C until all the methanol is gone.
The mixture will boil at some point between 100 and 60C. The melting point is similar to this. A mixture of methanol and water will freeze at some
point below 0C and above methanol's freezing point. Windshield washing fluid is a mixture of methanol and water. Ones that advertise to be good till
-20F are ~30% methanol I think. They will freeze completely at a point below 0C, even though there is water. Both of the components will freeze as a
mixture.
If water and the solvent are not miscible, then most of the water is pretty easy to separate. I am not sure but I suspect that the water remaining
dissolved in the solvent would not freeze out easily.
[Edited on 4/30/2017 by Geocachmaster]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Geocachmaster  |
"I don't know how do azeotropes act when they are cooled to the freezing points of their composants"
Azeotrope is defined as a mixture that cannot be separated or altered by distillation. The components will vaporize and condense with exactly the same
proportion and are thus unchanged. Azeotropes don't apply when the mixture is being frozen.
The problem is that mixtures of miscible liquids will have melting and boiling points that are an intermediate of the components. For example methanol
(bp ~60C) and water (bp 100C) are miscible with each other. A 50/50 mixture of methanol and water will not boil at 60C until all the methanol is gone.
The mixture will boil at some point between 100 and 60C. The melting point is similar to this. A mixture of methanol and water will freeze at some
point below 0C and above methanol's freezing point. Windshield washing fluid is a mixture of methanol and water. Ones that advertise to be good till
-20F are ~30% methanol I think. They will freeze completely at a point below 0C, even though there is water. Both of the components will freeze as a
mixture.
If water and the solvent are not miscible, then most of the water is pretty easy to separate. I am not sure but I suspect that the water remaining
dissolved in the solvent would not freeze out easily.
[Edited on 4/30/2017 by Geocachmaster] |
It's worse than that.
A mixture of methanol and water might have a melting point below that of either methanol or water.
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
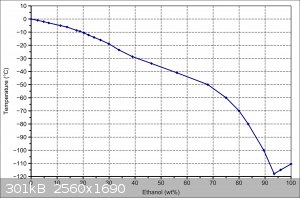
Oh yeah, ethanol too. This graph is from the Wikipedia ethanol page, you can see that ~94% ethanol will freeze below pure ethanol.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Geocachmaster  |
"I don't know how do azeotropes act when they are cooled to the freezing points of their composants"
Azeotrope is defined as a mixture that cannot be separated or altered by distillation. The components will vaporize and condense with exactly the same
proportion and are thus unchanged. Azeotropes don't apply when the mixture is being frozen.
The problem is that mixtures of miscible liquids will have melting and boiling points that are an intermediate of the components.
|
Only in the special case of solid solution.
All vapours are miscible with each other.
Some liquids are miscible with each other.
Some solids are miscible with each other - yet many substances which are miscible in liquid state are not miscible in solid.
Miscible liquids which are similar to each other obey Henry Law on evaporation. Meaning that they do have intermediate boiling point.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Geocachmaster  |
"I don't know how do azeotropes act when they are cooled to the freezing points of their composants"
Azeotrope is defined as a mixture that cannot be separated or altered by distillation. The components will vaporize and condense with exactly the same
proportion and are thus unchanged. Azeotropes don't apply when the mixture is being frozen.
The problem is that mixtures of miscible liquids will have melting and boiling points that are an intermediate of the components. For example methanol
(bp ~60C) and water (bp 100C) are miscible with each other. A 50/50 mixture of methanol and water will not boil at 60C until all the methanol is gone.
The mixture will boil at some point between 100 and 60C. The melting point is similar to this. A mixture of methanol and water will freeze at some
point below 0C and above methanol's freezing point. Windshield washing fluid is a mixture of methanol and water. Ones that advertise to be good till
-20F are ~30% methanol I think. They will freeze completely at a point below 0C, even though there is water. Both of the components will freeze as a
mixture.
If water and the solvent are not miscible, then most of the water is pretty easy to separate. I am not sure but I suspect that the water remaining
dissolved in the solvent would not freeze out easily.
[Edited on 4/30/2017 by Geocachmaster] |
Um, no.
A mixture will always freeze at a lower temperature than the pure substances. This is why you get melting point depression,so your organic compounds
are always checked for purity with their melting points.
Look at the phase diagram posted in a previous reply for ethanol and water. If you take a mixture of ethanol and water, say 30% wt ethanol, at room
temperature and cool it down, the phase diagram says that nothing will happen until -19 oC. At this point, ice will start to precipitate from the
mixture. This ice will be pure frozen water. If we cool it down to -30 oC, the water will continue to precipitate out as ice, and the remaining
liquid will be 40% ethanol by weight. Filtration at - 30oC will be difficult, so we won't get absolutely pure ice this way, but it can be used to
increase the concentration of alcohol (this is how they make apple jack, by the way. I've tried it, and filtering a slush is a pain in the
expletive).
If we cool it down to -70 oC, then enough of the water precipitates out that the remaining liquid is 80% ethanol. Once we get to -119 oC, that's the
eutectic, and everything freezes.
If we start with 99% ethanol and cool that down to between -110 oC and -119 oC, then solid ethanol will precipitate out, leaving a mixture of water
and ethanol in the liquid phase.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
alking
Hazard to Others
  
Posts: 252
Registered: 11-3-2016
Member Is Offline
Mood: No Mood
|
|
Whether it's feasible or not would depend upon the eutectic point of the solution as well as the solubility of the drying agent in the solvent you're
trying to separate, but perhaps with few exceptions I can't see why you would want to do this, what's the benefit? I can really only see doing it with
very hard to separate compounds either due to azeotrope formation or due to a very close boiling point such as acetic acid (in which case I would just
buy some GAA).
Freezing the solution uses just as much energy as distilling it, yet it's slower to occur if you're using a conventional freezer, you have less
control over it, and in many cases you're going to be left with both drying agent and water in your solvent. It just seems much easier to me to dry
> filter (and repeat as many times as necessary) and then distill. Your product will be superior in every way and very likely a comparable amount
of input involved.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by Geocachmaster  |
"I don't know how do azeotropes act when they are cooled to the freezing points of their composants"
Azeotrope is defined as a mixture that cannot be separated or altered by distillation. The components will vaporize and condense with exactly the same
proportion and are thus unchanged. Azeotropes don't apply when the mixture is being frozen.
The problem is that mixtures of miscible liquids will have melting and boiling points that are an intermediate of the components.
If water and the solvent are not miscible, then most of the water is pretty easy to separate. I am not sure but I suspect that the water remaining
dissolved in the solvent would not freeze out easily.
[Edited on 4/30/2017 by Geocachmaster] |
Um, no.
A mixture will always freeze at a lower temperature than the pure substances. This is why you get melting point depression,so your organic compounds
are always checked for purity with their melting points. |
A mixture will most of times freeze at lower temperatures.
Two exceptions:
Substances which are miscible in solid. In that case, the freezing point of mixture is indeed intermediate between pure compounds.
Substances which attract strongly. But that´s different between boiling and freezing.
If you have two strongly attractive substances, like water and a strong acid, then they form an azeotrope which boils higher than either pure
substance. But there usually is just one high boiling azeotrope, and the compositions between azeotrope and pure substance boil at temperatures
between azeotrope and pure substance. Also, the composition of azeotrope is not a small integer mole ratio, and it changes with pressure.
Such strongly attractive substances on freezing commonly form crystal solvates, such as hydrates. Crystal hydrates may freeze higher than either pure
compound, as is the case with hydrates of triflic and perchloric acids.
Important differences with azeotropes, though, are:
Unlike azeotropes, there may be several hydrates of different compositions.
Hydrates have fixed compositions with small integer mole ratios, and fixed crystal structure.
There are freezing point minima between hydrates and pure substances, and several hydrates. And such eutectic mixtures have non-integer compositions.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Having said all that, you can use freezing as a way to remove water (etc) from some solvents.
If you freeze most of a bottle of, for example, acetic acid, the water will be left behind in the last liquid (along with other junk) and you can
remove it with a pipette.
Works for cyclohexane, benzene, DMSO and presumably other solvents with accessible melting points.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
For anyone interested, the way apple jack was typically made, was by putting hard cider in a barrel half-buried in the ground during the winter, such
that it'd thaw a bit during the day, and freeze a bit during the night. The freeze/thaw cycles were what actually worked to separate the water from
everything else, (a lot like recrystallization) so if it was done perfectly, there would be a bunch of ice surrounding a core where everything that
wasn't water concentrated.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by chornedsnorkack  |
Such strongly attractive substances on freezing commonly form crystal solvates, such as hydrates. Crystal hydrates may freeze higher than either pure
compound, as is the case with hydrates of triflic and perchloric acids.
Important differences with azeotropes, though, are:
Unlike azeotropes, there may be several hydrates of different compositions.
Hydrates have fixed compositions with small integer mole ratios, and fixed crystal structure.
There are freezing point minima between hydrates and pure substances, and several hydrates. And such eutectic mixtures have non-integer compositions.
|
Hydrates are considered compounds, not mixtures.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | | For anyone interested, the way apple jack was typically made, was by putting hard cider in a barrel half-buried in the ground during the winter, such
that it'd thaw a bit during the day, and freeze a bit during the night. The freeze/thaw cycles were what actually worked to separate the water from
everything else, (a lot like recrystallization) so if it was done perfectly, there would be a bunch of ice surrounding a core where everything that
wasn't water concentrated. |
As I recall, you can get to about 40% ethanol in this way.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Latent heat of freezing is normally smaller.
Quote: Originally posted by alking  | | yet it's slower to occur if you're using a conventional freezer, you have less control over it, and in many cases you're going to be left with both
drying agent and water in your solvent. It just seems much easier to me to dry > filter (and repeat as many times as necessary) and then distill.
Your product will be superior in every way and very likely a comparable amount of input involved. |
If both substances are volatile then distillation will not, in principle, give a pure substance in a single step. You need many theoretical plates.
Again, in principle, growing large monocrystals of one pure substance does theoretically give a pure substance in one step. In practice, you have
viscous films of mother liquor, impurities trapped between crystals... but with distillation, you need multiple steps even in principle.
Also, freezing is at lower temperature, so unwanted decay reactions are generally slower.
|
|
|
alking
Hazard to Others
  
Posts: 252
Registered: 11-3-2016
Member Is Offline
Mood: No Mood
|
|
I assumed we're starting with an azeotropic mixture since OP is talking about drying water out of solvents so distillation without drying will not do
anything anyway, that's why I said to dry, filter, and then distill. The distillation is just to remove any drying agent so no need to use a column at
all. If your drying agent is insoluble in your solvent then there's no need to distill even, but then neither would there be to freeze so I assumed it
is or OP wouldn't be asking.
|
|
|