pharmastudent
Harmless

Posts: 4
Registered: 29-11-2016
Member Is Offline
Mood: No Mood
|
|
Need help with eugenol reaction
Hi,
I have a lab tomorrow but I'm confused about the reaction and what the chemical equation will be.
We're reacting eugenol with 4-chlorobenzoyl chloride and pyridine...I know we're trying to make a eugenol derivative and I think eugenol benzoate will
be formed. But I'm not sure what the chemical equation would be, or really understand the mechanism.
Can anyone help? 
[Edited on 30-11-2016 by pharmastudent]
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
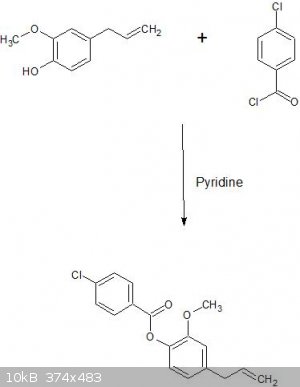
Yup, pretty sure eugenol 4-chlorobenzoate will be formed
Pyridine just serves as a base to pull the proton off the hydroxyl group on eugenol, forming the alkoxide ion which will then attack the electrophilic
carbonyl carbon on the 4-chlorobenzoyl chloride forming a tetrahedral intermediate. The chlorine will then leave when the electrons on the original
carbonyl carbon come back down to reform the C-O double bond.
I attached a picture of the overall reaction. Let me know if you need a diagram showing the mechanism in addition to me writing it out in words
[Edited on 30-11-2016 by JnPS]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
It's not going to be eugenyl benzoate unless the reaction is with benzoyl chloride. Eugenol is an alcohol (a phenol, IIRC, but still an alcohol).
Alcohols react with acyl chlorides to give esters. You should be able to find the general mechanism in your text, and come up with the specific one
for your set of compounds.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
pharmastudent
Harmless

Posts: 4
Registered: 29-11-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JnPS  | Yup, pretty sure eugenol 4-chlorobenzoate will be formed
Pyridine just serves as a base to pull the proton off the hydroxyl group on eugenol, forming the alkoxide ion which will then attack the electrophilic
carbonyl carbon on the 4-chlorobenzoyl chloride forming a tetrahedral intermediate. The chlorine will then leave when the electrons on the original
carbonyl carbon come back down to reform the C-O double bond.
I attached a picture of the overall reaction. Let me know if you need a diagram showing the mechanism in addition to me writing it out in words
[Edited on 30-11-2016 by JnPS] |
Thank you so much! 
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
@Draconic Acid, Damn it I thought I had this one, so my lovely drawn picture is wrong?
|
|
|
pharmastudent
Harmless

Posts: 4
Registered: 29-11-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | It's not going to be eugenyl benzoate unless the reaction is with benzoyl chloride. Eugenol is an alcohol (a phenol, IIRC, but still an alcohol).
Alcohols react with acyl chlorides to give esters. You should be able to find the general mechanism in your text, and come up with the specific one
for your set of compounds. |
Thank you!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No, it's correct. I just wanted to guide Pharmastudent to the right answer instead of giving it to him.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Ahh........., true.........guess I got overexcited, haven't been able to do any organic in a while
|
|
|
pharmastudent
Harmless

Posts: 4
Registered: 29-11-2016
Member Is Offline
Mood: No Mood
|
|
Thank you guys...I was on the right track but I wasn't 100% sure tbh, now its all fallen into place 
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Pyridine is more than a base here. It catalyzes the nucleophilic acyl substitution. I'm having trouble uploading the file, but google "Lies My
Sophomore Organic Professor Told Me" "Christopher R Jamison" and "Princeton" and you should find a helpful PDF. The first third of it discusses the
analogous acylation with dimethylaminopyridine (DMAP).
Pyridine is more nucleophilic than phenol, so Pyr first attacks the acyl chloride to displace Cl- and form an intermediate [acyl pyridinium]+[Cl]-
ion pair. This ion pair is setup to assist the phenol in the subsequent nucleophilic attack: a concerted deprotonation/substitution. In other words,
Cl- is the base. Not what you'd expect on the basis of pKa, but it agrees with computations and experiment. Pretty cool, yeah?
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
@DDTea is this the link you're talking about?
http://www.princeton.edu/chemistry/macmillan/group-meetings/...
That is pretty cool! Why do you think they keep teaching the old mechanism then? (I'm definitely bringing this up to my organic prof XD)
|
|
|
Nicodem
|
Thread Moved
30-11-2016 at 00:12 |
NitratedKittens
Hazard to Others
  
Posts: 131
Registered: 13-4-2015
Location: In the basket with all the other kittens
Member Is Offline
Mood: Carbonated
|
|
Quote: Originally posted by JnPS  | | Ahh........., true.........guess I got overexcited, haven't been able to do any organic in a while |
I know how you feel, I'm on an A-level chem course just waiting for organic chemistry to come around.
Basket of kittens for you ........BOOM
|
|
|