CoolFool
Harmless

Posts: 10
Registered: 30-4-2016
Member Is Offline
Mood: No Mood
|
|
Electrolysis of water
I wanted to show my sister how electrolysis of water works and so i filled a beaker with water and adder salt . I passed a current of 3 v . Some gas
was produced but to my surprise there was also formation of a brown precipitate. I checked the salt and found out that it also contained Potassium
Iodide . I carried it out again but the result was the same. Can someone tell me
the cause for this? . I carried it out again but the result was the same. Can someone tell me
the cause for this?
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
What are your electrodes made from? Could be corrosion
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
If your electrodes are steel or iron, that's almost definitely iron(III) hydroxide.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
CoolFool
Harmless

Posts: 10
Registered: 30-4-2016
Member Is Offline
Mood: No Mood
|
|
No I was using copper wires
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Then probably copper(I) oxide. Potassium iodide is harmless in this experiment and on top of that, its content in table salt is negligable.
Try stainless steel instead, or maybe carbon rods.
[Edited on 4-11-2016 by crystal grower]
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
At the anode you get impure copper(I) oxide. This stuff is yellow/brown.
At the cathode you get hydrogen gas.
If you use carbon rods (e.g. from spent zinc batteries), then you get chlorine at the anode, and you can easily smell it. That would be a more
understandable demo for your sister.
|
|
|
Texium
|
Thread Moved
4-11-2016 at 08:53 |
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
also, use sodium bicarbonate for the electrolyte as oxygen is slightly more electronegative than chlorine,
so elevtrolysis of sodium chloride solution produces H2 and Cl2 and NaOH in solution.
Most of the chlorine will combine with the NaOH but some escapes.
It just makes the explanation of the results easier using bicarbonate.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Matthew
Harmless

Posts: 6
Registered: 2-11-2016
Member Is Offline
Mood: No Mood
|
|
You might try getting the graphite out of the pencil. Don't heat up the rods too much though or they will break
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
You could also get a 6v battery and you'd get 4 carbon electrodes. Also I'd recommend using a high wattage. Remember P=I*E,
P-power in watts
I- ohms
E-Volts
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
To simplify the explanation, a baking soda solution using distilled water and using graphite electrodes would be best. That way you get oxygen and
hydrogen, and no complications from reactions with the electrolyte or electrodes.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Interestingly enough, I just found out that mild, low carbon steel is an excellent low-cost anode for water electrolysis. This is provided that the
Na2CO3 electrolyte is very pure, i.e., no halogen contamination. Just a few ppm of added NaCl is
enough to induce corrosion of the steel anode. With high enough electrolyte purity, however, I've used 1018 steel anodes in a cell for days with
little more than a slight color change from a transparent oxide layer. No coloring of the solution was noted, and no loose corrosion products were
present.
[Edited on 12-20-2016 by WGTR]
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Quote: Originally posted by WGTR  | | Interestingly enough, I just found out that mild, low carbon steel is an excellent low-cost anode for water electrolysis. This is provided that the
Na2CO3 electrolyte is very pure, i.e., no halogen contamination. Just a few ppm of NaCl is enough to induce corrosion of the
steel anode. With high enough electrolyte purity, however, I've used 1018 steel anodes in a cell for days with little more than a slight color change
from a transparent oxide layer. No coloring of the solution was noted, and no loose corrosion products were present. |
Wouldn't NaOH make for an even better electrolyte? No possible Co2 evolution, ofcourse provided it is pure.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
KOH also seemed to work, although that transparent oxide layer formed more quickly. It does seem to be less pure that the
Na2CO3, however. Here's what I used:
 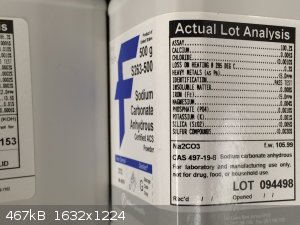
|
|
|
CoolFool
Harmless

Posts: 10
Registered: 30-4-2016
Member Is Offline
Mood: No Mood
|
|
I tried doing the same experiment with all the same stuff but this time i got a green precipitate . Is it copper hydroxide? also the current was 4.5V
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
It was probably Iron hydroxide. (Like what other people have said) I figured to say what I thought because with more information the better.
[Edited on 22-3-2017 by Liamatpm]
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
Quote: Originally posted by CoolFool  | | I tried doing the same experiment with all the same stuff but this time i got a green precipitate . Is it copper hydroxide? also the current was 4.5V
|
It was the copper like you said.
|
|
|