| Pages:
1
2
3
4 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Good news!
I have tried out the atm hydrogenation of dehydrorheosmin over Pd/C, using 25% mol/mol AcONa as an additive, in AcOEt, and after 14H all the
unsaturated ketone was reduced to the saturate dketone, without any traces of the alcohol!
I'm unsure if it is this substrate that is much more ressitant to reduction of the carbonyl, or if using sodium acetate is very effective as
suppressing 1,2-hydrogenation, but the reduction was text book perfect: regular H2 absorption, and theoritical amount consumed.
Using 1:1 AcOEt et ether as an eluant proved to give a superior seperation than
with the eluant used during the Zn reduction. I am unsure of which side-products are formed during the reduction, but I'm pretty sure Zn/AcOH can't be
used as a preparative procedure for phenolic butanones.. et ether as an eluant proved to give a superior seperation than
with the eluant used during the Zn reduction. I am unsure of which side-products are formed during the reduction, but I'm pretty sure Zn/AcOH can't be
used as a preparative procedure for phenolic butanones..
Sorry, still no photos I haven't got the camera issu dealt with yet..
Workup was simple: filtration of the catalyst and suspended AcONa, washing on the colorless filtrate, followed by drying and removal of the solvent..
The isolated product smells much nicer than the Zn reduction product! Much more subtle, "natural" aroma.
I can't wait to try the NiB hydrogeantion on the substrate. Even if it is clear than Pd/C is an ideal catalyst for such hydrogeantion, it si quite
expensive and not always accesable. A cheap, easily made catalyst would really be usefull to many.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
I'm unsure if it is this substrate that is much more ressitant to reduction of the carbonyl, or if using sodium acetate is very effective as
suppressing 1,2-hydrogenation, but the reduction was text book perfect: regular H2 absorption, and theoritical amount consumed.
|
The aliphatic ketone carbonyl is not reducible using Pd-C (at least not at the usual conditions), with or without additives such as you used. In
conjugated ketones it is normal that only the double bond gets reduced. Conjugated double bonds, particularly such conjugated with electron
withdrawing groups like in your case, are considerably slower to reduce than isolated double bonds. Actually, isolated double bonds reduce in matter
of minutes using couple of mol% 5% Pd-C at 1 atm. Moderately electron rich double bonds conjugated with the benzene ring (aka styrenes) also reduce
quite fast.
Thanks for sharing your experience with Cu/SiO2 catalyst. I'm looking forward to read about your experiments using nickel boride based catalysts.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, when reducing dehydrozingerone, at least 5% saturated alcohol was formed using Pd/C and K2CO3 (which I neutralized after). The Chem Educ.
article on Rheosmin also mention this. It seems dehydrorheosmin is much less prone to this.
One of the NiB article claimed 99% selectivity using Pd/C, which i doubt at first, but has now confirmed. They say NiB give sthe same selectivity, but
with a much higher absorption rate, and reduction of the double-bond is doen in a matte rof minutes. Can't wait to get this started.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
That ref I gave over in the Cassione, using MVK in a Heck reaction, used Pd/TiO2 for both the Heck and the reduction of the C=C double bond, further
suggesting that Pd is useful although perhaps not optimal time-wise for this class of reduction. I wonder if Pd/TiO2 would prove easier to reactivate
than Pd/C.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Ok, I just HAD to find a camera to take pictures of theses and show them to you guys... I have fallen in love of my Rheosmin and have been showinbg
it to evryone I cross 



96.6% Yield by H2/ Pd/C hydrogenation (actually I realized absorption was finished after 4h, as the theorical volume was 150mL! I guess the other
100mL were lost from small leaks or something. The absorption rate had greatly diminished afetr 150mL mark...
Ideal TLC eluant: 50:50 AcOEt: Pet ether. Will get Rf if someone wants them (saturated ketone higher than the unsaturated one, no traces of alcohol or
other by-products!)
Let's hope NiB can do as good! I feel like making dozen grams of the stuff, my room smells beautifull  Pretty strangely, the nice crystals smell much less than the crude powder, before recrystallization. Pretty strangely, the nice crystals smell much less than the crude powder, before recrystallization.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I've stumbled on a pretty interesting article, detailing the use of NiB supported on silica gel to selectivily reduce cyclopentadiene to
cyclopentene...
NiB/SiO2 is prepared by impregneting NaBH4 on silica gel, then adding this silica to a solution of NiCl2, forming amorphous NiB on the support. Good
selectivities are reported for the reduction of cyclopentadiene, and I assume the NiB posses the same reactivity as collodial NiB towards unsaturated
ketones, and would then offer a good alternative to collodial borides and their tedious workup..
EDIT: attached article
Selective hydrogenation of cyclopentadiene to cyclopentene
Wang et al.
Applied Catalysis A; 163, 101-109 (1997)
Abstract
An amorphous NiB/SiO2 catalyst, with a large specific surface area, was prepared by a reductive-impregnation method. The
selective hydrogenation of cyclopentadiene to cyclopentene was carried out in a continuous flow fix-bed reactor at
atmospheric pressure and with 10 g g cat -I h -l of cyclopentadiene feed. The catalyst showed high selectivity and stability.
Cyclopentene was obtained in 96--100% yield at complete conversion of cyclopentadiene at temperatures ranging from 80°C
to 200°C and no significant decrease of the activity was observed during the reaction period of 500 h. The catalyst sample was
characterized by ICP, XRD, DSC, SEM, XPS, BET and 02 adsorption. XRD measurement revealed that the amorphous state
was kept after catalytic reaction. Differential kinetic study showed that the hydrogenation proceeded according to a Rideal-
Eley mechanism.
Keywords:
Supported amorphous alloy; Nickel-boron alloy; Cyclopentadiene hydrogenation; Catalytic activity; Differential
kinetics
[Edited on 6-2-2009 by Klute]
Attachment: NiB_SiO2.pdf (590kB)
This file has been downloaded 1905 times
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
stateofhack
Hazard to Others
  
Posts: 123
Registered: 6-5-2008
Location: Warm Coast
Member Is Offline
Mood: annoyed
|
|
Yes i am !
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Klute you have some amazingly pretty crystals there! Nice work and great yield!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you 
They really are part of my favourites! Both the smell and the apparence!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Recently I have got few grams of 10% Pd/alumina, so I have tried hydrogenation of benzylideneacetone. Unfortunately, suspension of catalyst (0,3g) in
30g unsaturated ketone+50 cm3 of toluene does not want to "eat" hydrogen (under almost atmospheric pressure and 30-40 C). I also tried ethyl acetate
instead of toluene - results the same.
This Pd/alumina is a little old - is it possible that it needs to be activated somehow ? I mean H2 stream nad ~300 C....
I do not know, I am not familiar with hydrogenations on Pd 
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Klute has reported in this thread that formate can liven up an old catalyst. Can't say about the solvent, but I would stick to GAA for starters.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
The activity of the catalyst depends on how the metal has been deposited on the carrier. There are three types of deposition and the one suited for
low pressure reactions is the eggshell type. The fact that alumina is the carrier makes it likely that you have an industrial catalyst which requires
higher pressures, although there are eggshell types of catalysts using alumina as carrier.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
All I know is on the label: Koch-Light, 8874h Pd 10% on alumina, for lab use only.
I did reactivation in slow H2 stream at 320 C and hydrogenation takes place, but is very slow. I will try to increase overpressure from 100 to 200 mm
of H2O.
ps. product smells nicer and nicer, strawberry-like and reminds me benzylmethyl ketone.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I would try using CTH conditions, I requested a few articles on the subject lately, apparently ammonium formate gives excellent resultd for the
selective reduction of the conjugated double bond, without producing any (or very little) alcohol. You could try adding some formate to a suspension
of your catalyst and see if any gas is evolved, then try the reduction under CTH conditions in methanol. Good luck! Please let us know how it goes!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sydenhams chorea
Harmless

Posts: 29
Registered: 16-8-2009
Member Is Offline
Mood: No Mood
|
|
Why not give sodium dithionite a try for the reduction of the double bond?
It works for isatylideneacetone, it might as well for your substrate. The procedure is so straightforward that it can't hurt giving it a try.

Tet. Lett. 49, 21 p 4741-4758 (1993)
Attachment: isatylideneacetones.pdf (1.8MB)
This file has been downloaded 1221 times
Il n'y a point de sots si incommodes que ceux qui ont de l'esprit.
François de La Rochefoucauld.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Interesting, never thought of that. Could be worth a try!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sleipnir
Harmless

Posts: 8
Registered: 10-9-2009
Member Is Offline
Mood: No Mood
|
|
Maybe you could try a catalytic reduction with an iron catalyst:
catalytic amounts of [Fe(CN)5NH3]3- reducte in an excess of NH2OH oelfines without affecting carbonyl functionalities and aromatic rings
[Fe(CN)5NH3]3- can be prepared from [Fe(CN)5NO]2- (Nitroprusside-anion) with can be prepared from [Fe(CN)6]4-
seen at: http://pubs.acs.org/doi/abs/10.1021/jo800302v
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Interesting alternative, but the preparation of the catalyst requires cyanide, which I do not have, and isn't specialy oTC.. But surely other ligands
could work aswell..
I have started to interest myself to homogeneous catalysis, using avaible transition metals, like Cu, Fe, Ni, etc. Not alot of ligands are easily made
at home, but there could be some viable paths.. I guess reduction-wise , Nickel complexes are the best candidates. If I could find a cheap source of
ruthenium, I could try using some of teh catalysts I worked on recently, very active in CTH conditions..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
No, it does not require cyanide. Potassium ferrocyanide , K4Fe(CN)6.3H2O is very cheaply available from chemicals suppliers, is not suspious, and is
not toxic. The cyanide is only slowly released when heating with a strong acid.
IIRC, the nitroprusside can be prepared by treating the ferrocyanide with nitric acid, but I don't know the exact procedure. Be warned, the
nitroprusside is very toxic, at least it is when injected.
|
|
|
Sleipnir
Harmless

Posts: 8
Registered: 10-9-2009
Member Is Offline
Mood: No Mood
|
|
other lingand no problem.... 
http://pubs.acs.org/doi/abs/10.1021/om050625b
but i think this one might be carcinogen and it need's (silght) overpressure... would prefer the [Fe(CN)5NH3]3- which possibly is not toxic at all
since the toxic effekt of the [Fe(CN)5NO]2- is induced by the elimination of NO
I've tryed to make [Fe(CN)5NH3]3- some time ago (wanted to use it on dehydrozingerone) but instead of obtaining nice red crystals i obtained a brown
slurry, if someone made this already i would be thankfully for some hints ^^
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Sydenhams chorea  | Why not give sodium dithionite a try for the reduction of the double bond?
It works for isatylideneacetone, it might as well for your substrate. The procedure is so straightforward that it can't hurt giving it a try.

Tet. Lett. 49, 21 p 4741-4758 (1993)
|
Interesting. However, that double bond is doubly alpha to carbonyls, and hence highly activated. Perhaps thiourea dioxide would be worth a test here.
EDIT: What am I thinking. Thiourea dioxide would reduce the ketone.
[Edited on 9-14-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Here's a little something for anyone interested. I ran the condensation of Vanillin with acetone (24h at room temperature), and recrystallized my
product from 50:30 water:isopropanol. I then brought it over to school for use as a Michael acceptor in undergrad research. Here's a 400MHz proton NMR
for you guys. Looks pretty good for all over the counter chemicals, huh? 
The peak at 1.6ppm is water, but after running a blank of CDCl3 through the NMR, it's contamination of the chloroform, not the sample.
2.35ppm is the methyl ketone.
3.9ppm is the methoxy group
5.9ppm is the phenol hydrogen
The "triplet" with integration of 2 at 7.1ppm is the overlap of the singlet from the hydrogen in the 2-position on the ring with what I suspect is the
6 position hydrogen's doublet.
Unrelated, but has anyone seen what this stuff costs from Aldrich?
http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en...

[Edited on 9-23-09 by UnintentionalChaos]
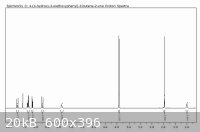
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Nice! Thanks for sharing! It's a rather clean spectra you've got there! Looks like the reaction is pretty selective and no impurirites remain after a
single recrystallization..
Do you intend on reducing it to the butanone? A spectra of the butanone would be great too!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Klute  | Nice! Thanks for sharing! It's a rather clean spectra you've got there! Looks like the reaction is pretty selective and no impurirites remain after a
single recrystallization..
Do you intend on reducing it to the butanone? A spectra of the butanone would be great too! |
I also have a carbon spectra for this one if you'd like and I forgot to mention that I took a m.p. with a thiele tube and got 126-128C.
Possibly over winter break when I'm at home, but not with this material. More than likely, it'll be O-methylated to shield the phenol, since we think
the reaction we're studying proceeds by a radical mechanism and phenols are radical traps. I won't know until I try though.
I will hopefully prepare some of the 4-hydroxyphenyl variant and reduce it to rheosmin, which I can get spectra for.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
somebird
Harmless

Posts: 1
Registered: 29-7-2015
Member Is Offline
Mood: No Mood
|
|
Thanks for Klute's thread, i learn a lot from here. And the pink colour must be the combination phenol and zn2+ . Maybe add EDTA solution can
eliminate it.
|
|
|
| Pages:
1
2
3
4 |