CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
chlorination of benzene
is possible to do chlorination of benzene with AlCl3 without any special condition?
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Depends on how do you define "special condition".
It does not require microwaving, sonication, UV, VIS, IR, etc. lighting, no high-voltage discharge either.
But does require anhydrous reagents because AlCl3 is water sensitive.
The emitted HCl gas can cause unease in a (shared) kitchen environment, so proper venting is also useful.
Might require temperature control, depending on mass of materials used.
Why not consult e.g. Vogel or maybe Org.Syn. for a proper description of the process. Wait! They may not use AlCl3 but FeCl3
instead (or something similar), the quirks are roughly the same.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Here's what I found
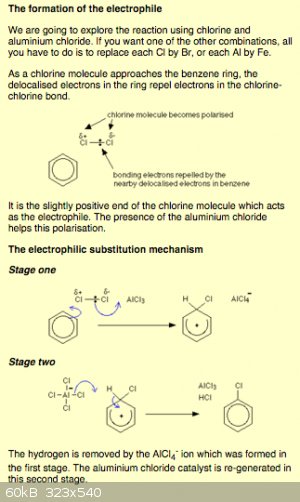
[Edited on 11-19-2015 by Detonationology]
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
and is possible to chlorinate toluene to benzyl chloride react it with potassium cyanate and chlorinate benzyl cyanide in pozition 4?
[Edited on 22-11-2015 by CrossxD]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Hmm I have a big bag of FeCl3... I bought it for something else (and only needed a small quantity), but it might be fun to put some groups onto
benzene rings.
|
|
|
kecskesajt
Hazard to Others
  
Posts: 299
Registered: 7-12-2014
Location: Hungary
Member Is Offline
Mood: No Mood
|
|
But you could easily chlorinate with TCCA! Discussed on the site several times.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by CrossxD  | and is possible to chlorinate toluene to benzyl chloride react it with potassium cyanate and chlorinate benzyl cyanide in pozition 4?
|
You'd need KCN, not KCNO.
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JJay  | | Hmm I have a big bag of FeCl3... I bought it for something else (and only needed a small quantity), but it might be fun to put some groups onto
benzene rings. |
If it is in a bag then chances are it is unsuitable for this purpose. Not because of the packaging material but I doubt that anhydrous ferrichloride
would survive in a plastic bag too long. If it is orange colored then it is unsuitable (hydrated). If the color is black and the powder is obviously
dry, then you are lucky.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's black and appears dry.... I keep it triple bagged.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Adding some degreased steel wool is quite sufficient to form fresh, high-surface area FeCl3 in-situ. It works well with benzene bromination and I'm
sure it would work with chlorination (though I personally loathe working with gaseous chlorine).
[Edited on 23-11-2015 by UC235]
|
|
|