| Pages:
1
2 |
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
Assistance Needed to Balance Equation to Determine Theoretical Yield
I am new to this forum but not new to amateur experimentalism. I took college chemistry once upon a time and but much of the more basic material
eludes me. I have a synthesis that I would like to evaluate the efficacy of, which I already know to be poor, so that I may attempt to improve upon
its' values. More importantly, I want to UNDERSTAND exactly what is taking place. I am not asking simply for the solution (no pun intended) but an
explanation of the processes (what are the reaction products and how I would balance it) leading up to it alongside this answer. Any help would be
greatly appreciated. And before anyone gets upset, I did make a solid attempt at figuring this out myself and I feel that I need someone to explain it
to me. Much like a student might have a textbook with all the answers but a teacher is also present because oftentimes a different perspective is
required.
In the synthesis, solution A is added dropwise to solution B allowing for an ether to transform into an ester to for use of the 1deg alcohol oxidation
into a lactone.
Solution A: (CH2)4O + CH3CN + CH3CO2H
Solution B: Ca(ClO)2 + H2O
If you understand this equation then you'll almost certainly understand its ultimate purpose as it has limited use for anything else. However, it
bears no importance to my inquiry. I am seeking a complete understanding of the chemistry involved here. Thank you! :-D
[Edited on 16-2-2015 by RelativeEffectiveness]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
No one will help you make drugs. There are plenty of other substrates for this reaction if you are interested in the chemistry, choose one of them.
There seem to be a lot of people trying to synth GBL recently.
|
|
|
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
I know how to make it. I can make it. That is not the point. I want to know more intimately the actual chemistry taking place because it interests me.
I am asking for help solving and balancing the equation. I dont "need anyones help making drugs." I need someones help better understanding chemistry.
If it is better for your conscience, provide me with a similar substrate with similar or identical reactants and go through it for me.
Lets use tetrahydropyran instead then.
[Edited on 16-2-2015 by RelativeEffectiveness]
[Edited on 16-2-2015 by RelativeEffectiveness]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Then why choose this substrate if you are just interested in the chemistry? If you really want to know, search, hypochlorite oxidation of legal
substrates is a common lab experiment.
|
|
|
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
Because I did not know enough about chemistry to know that this was such a common reaction. I apologize. I was simply using that reaction because I
was familiar with it. If you could attempt to look past the idea that i am trying to make drugs and replace it with your new understanding of my
desire to comprehend solving and balancing this type of reaction I would still appreciate an explanation from anyone will.
[Edited on 16-2-2015 by RelativeEffectiveness]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RelativeEffectiveness  | | If you could attempt to look past the idea that i am trying to make drugs and replace it with your new understanding of my desire to comprehend
solving and balancing this type of reaction I would still appreciate an explanation from anyone will. |
And how is anybody to do that? You forgot to give the reference and without it there can be no reasonable answer.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
This question makes no sense - you have to specify what you want the yield based on. THF or Ca(ClO)2?
There are many things wrong with your post. Don't beat around the bush. Give references (https://www.erowid.org/archive/rhodium/chemistry/ether2ester...). Give a clear title (e.g. Stoichiometry of calcium hypochloride oxidation of THF
to gamma-butyrolactone). Ask your question clearly and succinctly. Sensitivities go to Whimsy.
Anyway, the classic high-school solution to the question would be:
(1) THF + H2O ---> GBL + 4H+ + 4e-
(2) Ca(ClO)2 + 4H+ + 4e- ---> CaCl2 + 2H2O
(1) + (2) ==> THF + Ca(ClO)2 ---> GBL + CaCl2 + H2O
PS: GHB sucks. IMHO it's on the same level as alcohol. Wasting acetonitrile on this reaction is a bit of a sacrilege.
| Quote: | | No one will help you make drugs |
Who gives you the authority to speak for everyone? Stop being a prick.
[Edited on 17-2-2015 by turd]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by turd  |
| Quote: | | No one will help you make drugs |
Who gives you the authority to speak for everyone? Stop being a prick.
[Edited on 17-2-2015 by turd] |
You're right, I phrased that wrong. Everyone who values their equipment and doesn't want to face the small chance of being charged as an accomplice
will not help you make drugs. I apologize.
[Edited on 2-17-2015 by gdflp]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Luckily, there are many college chemistry textbooks, study guides, and lecture series readily available online. Read them.
|
|
|
j_sum1
Administrator
       
Posts: 6321
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Balancing equations and determination of yield are pretty fundamental skills.
Generally this is learned in simple experiments like acid-base neutralisation, simple redox, electrolysis of water, combustion and that kind of thing.
If you are refreshing on these skills and require help, I have no idea why you would choose this as an example.
If you are investigating a reaction mechanism then I am not sure why you would ask about yield nor again why you would choose this particular example.
If you are wanting a leg-up in your new enterprise as a drug cook, I am not sure why you would ask here. This is not the focus of this board.
So, I am confused by your question.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  |
This question makes no sense - you have to specify what you want the yield based on. THF or Ca(ClO)2 |
Hardly. The question may not make sense for reasons others have stated, but your comment here is not one of them. Obviously in a balanced equation,
the theoretical yield is 100%. In practice, unless otherwise stated, absolute yield is generally understood to be by mass (rather than molar yield)
and percent yield is in terms of the limiting reagent unless specified. This does make experimental yield determination without quantified
data meaningless, but that isn't what the poster asked and not for the reason you're stating while you're calling members unnecessary names instead of
just disagreeing with them which is pretty clearly against forum decorum, you can look it up or ask a chemist if you disagree.
http://orgchem.colorado.edu/Technique/Procedures/Notebook/Yi...
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The question may not make sense for reasons others have stated, but your comment here is not one of them. |
Yes, actually it is - if the yield is supposed to be on THF there is no need to "balance" an equation for determining yield:
THF + x oxidizer ---> GBL + y something
If the yield is supposed to be on the oxidizer things may get more hairy, because oxidizers may behave differently in different conditions.
| Quote: | | In practice, unless otherwise stated, absolute yield is generally understood to be by mass (rather than molar yield) and percent yield is in terms of
the limiting reagent unless specified. |
Thank you Mr. Wikipedia-Chemist. Now can you also post something useful and help OP?
[Edited on 17-2-2015 by turd]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  |
Yes, actually it is - if the yield is supposed to be on THF there is no need to "balance" an equation for determining yield:
THF + x oxidizer ---> GBL + y something
If the yield is supposed to be on the oxidizer things may get more hairy, because oxidizers may behave differently in different conditions.
|
You are making the assumption that your x coefficient is in excess of the understood '1' in front of THF for some reason, assuming one molecule of
oxidizer per THF molecule in the balanced equation (necessary to normalize to). This assumption is a mistake.
A percent yield only makes sense with regard to a theoretical yield, which is what the OP asked about. Read what he said. You need a limiting reagent
from a balanced equation to determine this, end of story. If you disagree, you are obviously just completely ignorant or trolling.
How exactly do you propose someone determine the limiting reagent without a balanced equation, then? This is part of what the OP wants as far as I
understand "I have a synthesis that I would like to evaluate the efficacy of, which I already know to be poor" and then asking for... a balanced
equation, presumably so they can determine the denominator of their percent yield for comparative purposes under what are presumably different
reaction conditions for comparative optimization in a method oriented synthesis in synthetic methodology, as opposed to something like total
synthesis. I know I haven't been in a chemistry class in awhile, but it appears to still be taught the way it was in every course I ever took, and how
I had to keep logs for experiments in bound notebooks in research and in publication. I guess I just lack all the experience tard here has, er, turd.
My apologies, your calling me an armchair chemist doesn't mean I should insult you back, so now that we have exchanged barbs, how about address what
the poster said, or the content of my post rather than making wild assumptions and ignoring how yield conventions work.. and/or why don't you go brush
up on how a yield is determined like I suggested.
Determining "yield on the oxidizer" when your limiting reagent is THF is meaningless in terms of theoretical yield. No one publishes yields like this.
Feel free to find me a peer-reviewed article where someone has just to show me wrong. Your theoretical yield is limited by the limiting reagent, hence
the name. The limitation is stoichiometric, which requires some form of equation balancing. No one determines a theoretical yield by a reagent in
excess, regardless of whether that excess increases percent yield.
| Quote: | | Thank you Mr. Wikipedia-Chemist. Now can you also post something useful and help OP? |
Useful like you're
being as you level insults at everyone while ignoring the original poster's actual questions and being arrogant in... what, speaking for the OP?
Didn't you just criticize someone for speaking for others? Gee, if an .edu domain website on organic chemistry lab notebooks and yields, like the OP
mentioned in their post, isn't helpful I don't know what is. Then again, you clearly ignored it, then went to wikipedia and found that it too says
what every textbook on chemistry says and ignored that too, so I guess that's indicative of something. If someone asks for a total yield, it's assumed
they mean by mass unless they state "molar yield." Similarly, it's understood that a percent yield is with respect to a limiting reagent, which you
implicitly determine relative a balanced reaction equation. This doesn't have to be specified, but it does have to be done.
Maybe if you were educated in the subject you could use the proper terms such as "limiting reagent" or distinguish theoretical from percent yield
while you go calling people 'wikipedia chemist' and citing solely from erowid mirrors. In fact, take said article. Your source. Note how the
comparative yields per oxidizer are listed? Those are percent yields with a denominator of the theoretical yield given a limiting reagent, tabulated
solely for comparative purposes across substrate. The percent yields are still normalized based on the theoretical yield, which is based off the
limiting reagent. If they weren't done in this manner, how would you compare them? The hubris is matched only by your ignorance. I would expect
someone working from wikipedia, or the academic educational source I cited, to know the difference between a percent and theoretical yield, which is
more than I can say for your posting at the moment. Maybe you can cite your own source that disputes how a theoretical yield is determined, and
enlighten us all, or stop insulting people and admit you made a mistake.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Serious question: Have you been diagnosed with autism / Asperger's? That would explain your incoherent rants on complete trivialities.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | | Serious question: Have you been diagnosed with autism / Asperger's? That would explain your incoherent rants on complete trivialities.
|
Nah, but I play autism spectrum on the internet. By the way, claiming that balancing a reaction is not necessary to calculate a theoretical yield is
the kind of triviality that fails students straight out of chemistry, and (autism mode) is against the Sciencemadness Guidelines (TM).
Serious question back at you: are you trolling, or just prone to making what you feel are completely trivial mistakes (dyslexia?) while insulting
people? Honest question. Because that would explain how much difficulty you express at simple, reiterative English making sense.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
I may be dyslexic, but you clearly lack in even the most basic understanding how reaction equations work. E.g.:
| Quote: | | You are making the assumption that your x coefficient is in excess of the understood '1' in front of THF for some reason, assuming one molecule of
oxidizer per THF molecule in the balanced equation (necessary to normalize to). |
This makes no sense. x is determined by the reaction. It cannot be in excess of something. You don't write unreacted reagent to the right of the
equation. And that was only the first sentence of your rant!
And no, in many cases a chemist does not have to balance equations to calculate yields. It's not uncommon to have different reactions at the
same time. Example: You oxidize a substrate with KMnO4. As long as you use an obvious excess of KMnO4, it's irrelevant whether it goes down to MnO2 or
Mn2+ or some crazy mixture, if you calculate the yield on the substrate.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  |
You have 'x' as a reactant coefficient for your oxidizer on the LEFT of your equation. It absolutely can be in excess of the limiting reagent if this
is not a balanced equation. |
Again: you don't understand what an equation is.
x is defined in what I have written above. It cannot be in excess.
| Quote: |
Your entire argument was based on not needing to balance equations for determining yields, when the poster was very specific about percent yield. I
already explained percent yield does require a balanced equation.
|
No, it doesn't and in some cases there is no well defined equation. You will find many papers without equations, yet calculated yields.
| Quote: | | Now we're getting into semantics. How does a chemist know what an excess is unless they mentally ballpark a balanced equation to some arbitrary order
of magnitude? |
From experience. E.g. you cook phenylacetone from phenylacetic acid in acetic anhydride. You use a huge excess of acetic anhydride, because you know
that you will get biaryl product otherwise. Say 20 g PAA in 800 ml AA. You can calculate yield based on PAA without knowing how much AA reacts with
PAA.
| Quote: | | As for multiple reactions occurring at once, that's irrelevant. |
Absolutely not. Your oxidizer can have different pathways to different oxidation states.
You cannot determine what you get in the end, therefore you cannot balance your equation, still you can determine your yield based on substrate. QED.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | | I may be dyslexic, but you clearly lack in even the most basic understanding how reaction equations work. E.g.: |
Hardly close to reality with that assumption about my understanding. Balanced reaction equations have nothing to do with unreacted reactants... those
are equilibrium constants. You seem to be conflating the two, or assuming I was discussing them. I was not.
| Quote: | | This makes no sense. x is determined by the reaction. It cannot be in excess of something. You don't write unreacted reagent to the right of the
equation. And that was only the first sentence of your rant! |
This isn't relevant to anything I said. Maybe you didn't understand what I said, but I am not under the impression you write unreacted reactants on
the right of a balanced equation, nor did I state one should.
You have 'x' as a reactant coefficient for your oxidizer on the LEFT of your equation. It absolutely can be in excess of the limiting reagent if this
is not a balanced equation. Your entire argument was based on not needing to balance equations for determining yields, when the poster was very
specific about comparing reactions, which requires percent yield. I already explained percent yield does require a balanced equation, and theoretical
yield (hence why the original poster is asking, I presume).
| Quote: | | And no, in many cases a chemist does not have to balance equations to calculate yields. It's not uncommon to have different reactions at the
same time. Example: You oxidize a substrate with KMnO4. As long as you use an obvious excess of KMnO4, it's irrelevant whether it goes down to MnO2 or
Mn2+ or some crazy mixture, if you calculate the yield on the substrate. |
Now we're getting into semantics. How does a chemist know what an excess is unless they mentally ballpark a balanced equation to some arbitrary order
of magnitude? Nowhere did I state balancing couldn't be mentally estimated, or that one needed a certain number of significant figure: just that it
had to be done. Now it would seem we're agreeing in principle, but seemingly conflicted about methodology.
As for multiple reactions occurring at once, that's irrelevant. You can have all kinds of spectator ions on both sides of an equation if you wanted,
though it's not simplified, and ping-pong kinetics in reactions are also possible to write, but the level of specificity has to allow you to balance
your product and reactant to determine a theoretical yield, which is what the poster was asking about!
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Oops. Deleted my post trying to reply. Hit back and posted out of sequence above.
Quote: Originally posted by turd  |
Again: you don't understand what an equation is.
x is defined in what I have written above. It cannot be in excess.
|
I beg to differ. You don't seem to understand what a variable is, and you're questioning my grasp of equations. Nowhere did you define x that I see.
Please point that out explicitly for me in case I missed it. In your original post there, which I quoted, you have:
"Yes, actually it is - if the yield is supposed to be on THF there is no need to "balance" an equation for determining yield:
THF + x oxidizer ---> GBL + y something
If the yield is supposed to be on the oxidizer things may get more hairy, because oxidizers may behave differently in different conditions."
Where is x defined? There is no mathematical (or physical, more importantly) reason that x can't be between 0 and 1, which makes the oxidizer the
limiting reagent if it is only capable of oxidizing one equivalent of THF each, which makes a theoretical yield in terms of THF meaningless. If you
said x>=1, x= some number greater than or equal to one, or even (1+x), there would be clear excess regardless of whether your oxidizer oxidized 1
or 20 equivalents of THF. You went back and edited in a balanced set of half reactions in your earlier post now, without mentioning, I see. This
wasn't there originally. Why do it if it were completely unnecessary for the poster?
| Quote: | Absolutely not. Your oxidizer can have different pathways to different oxidation states.
You cannot determine what you get in the end, therefore you cannot balance your equation, still you can determine your yield based on substrate. QED.
|
Pathways aren't shown in a balanced equation, nor are transition states or a lot of other irrelevant things to theoretical yield calculation. And yes,
you can determine what you get in the end of a reaction. It's called characterization, and it honestly kind of sucks. HPLC, UHPLC, GC/MS, and various
other chromatographic separations and spec techniques give a very good idea of what you get in a reaction. You might not 'know' it before your
experiment, but that is not relevant to theoretical yield, which is the only yield in the thread title/original post!
A pathway doesn't affect a theoretical yield for identical limiting reagent molar quantities.
Different pathways to a reaction, or papers without balanced equations written, still presumptively balance the equation the authors want to happen,
or expect, in terms of calculating percent yields from limiting reagents and theoretical yields. That is where the "theory" portion of the yield comes
from. The actual equation might not be printed in the paper or the supplementals, but it's discernible from the reaction schemes and molar
equivalences. Published scientists don't generally post every little calculation or bit of data they did when it's common knowledge and can be
determined from what they do write, even though it may have been done. Ask anyone you can verify has published and trust, since clearly you want to
pretend I just don't understand equations without linking to anything that supports where you were taught your paradigm with regard to theoretical
yield.
Now you're discussing how efficient or clean a reaction is, as side reactions or intermediates may form. Again, this is actually irrelevant to
determining theoretical, though as I said earlier, may affect the percent yield. I was very specific about this. Look at any complete combustion
reaction of fuel and oxygen to CO2, H2O. That is an idealized scenario, yet it is still used for calculation of BTUs. I have personally synthesized
and painstakingly characterized reactions under various conditions where yields improved, but side products increased. In one reaction condition, very
pure products and unreacted substrate were separated. In a second condition, more product, less reactants, and various additional side products were
made. As you said, not uncommon. Yield was determined identically for comparisons.
As for experience, that is still mentally ballparking a balanced equation. You have to have some comprehension of what the limiting reagent is. This
is a mental calculation. If you don't like my terminology, fine. You don't consider it a calculation, even if instinctual, to determine the limiting
reagent. Clearly an experienced chemist working from memory or intuition has enough of a grasp of the relative molar quantities of two reactants
interacting to get a known product and balance an equation in their heads. It might not be simplified, but it was done at some point, even if
instinctually. The OP is obviously not there, nor is that what they asked. Changing topics to "cooking" P2P might be an entirely different
matter, as I don't necessarily expect a "cook" to even care about yields, waste disposal, etc.
[Edited on 18-2-2015 by Chemosynthesis]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I had some time to waste and felt like playing around in ChemDraw anyway. If you really are interested in the actual chemistry involved in
that reaction, I'm guessing this is how it probably works. The THF gets oxidized twice, with the second oxidation proceeding through either a
hydroxyaldehyde or a cyclic hemiacetal (probably both). Any input from others is welcome.
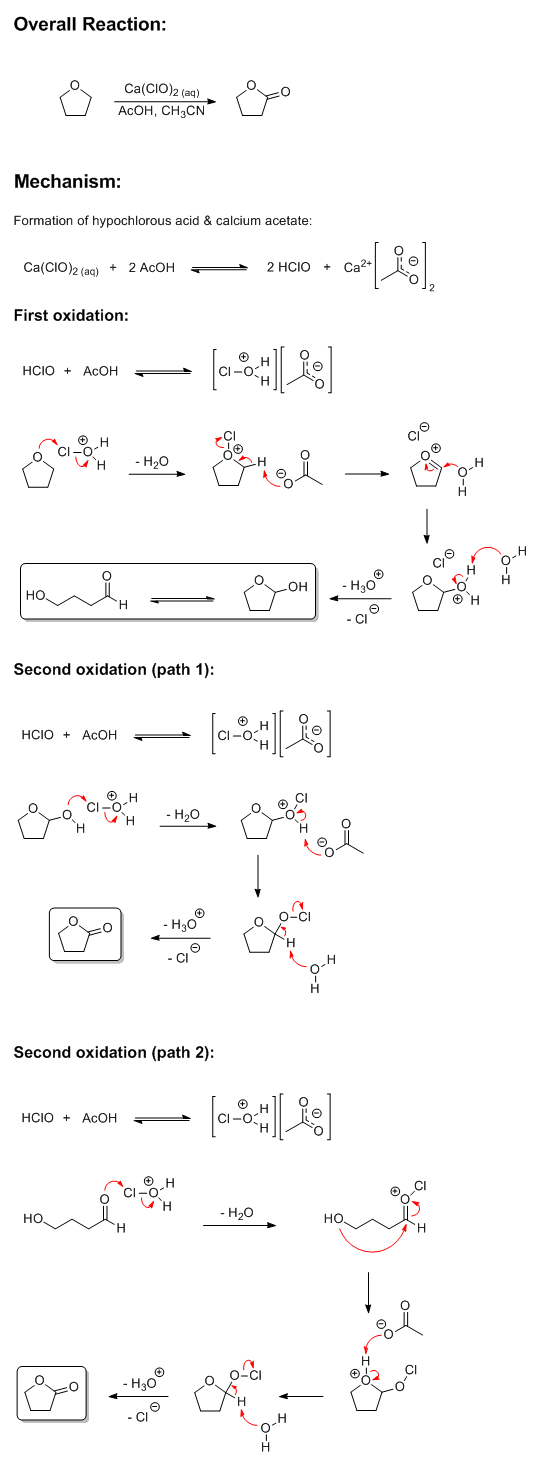
[Edited on 2-17-2015 by Darkstar]
|
|
|
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
Thank you everyone for your input. This is the actual reference. I had chosen this reaction because I am familiar with it and it interests me. Can I
not choose to practice and relearn the fundamentals of chemistry on a substance that has value as a recreational drug? You all assume that mynintent
is to use this knowledge to pursue clandestine and illicit experiments. The isn't the case. Been there done that. Im genuinely interested in
understanding a reaction that I have performed in the past as my interests have become more fixated on the chemistry itself rather than the drug. I
would hope someone can understand that without an immediate bias against drug synthesis and the connotations they elicit.
I am also aware of the existence of chemistry textbooks. After looking through much of auch information I opted to ask the opinions and look to see
the insight of like-minded chemists with substantially more knowledge and experience than myself. I assumes this was a community of chemists
interested in the chemistry of all organic and inorganic materials. If you do not wish to help me, than abstain from doing so. But please do not
patronize me with sarcastic remarks about textbooks and how I would have been better off never asking this question or for help in this forum. This is
counterproductive. For those who have not misinterpreted my intent, I thank you for your input.
"A general procedure is outlined for the oxidation of l-menthol to l-menthone. Thus l-menthol (3g, 19mmol) was dissolved in acetonitrile : acetic acid
(3:2 25ml) and added dropwise over a period of ten minutes to a cooled (0degC) and stirred solution of Ca(OC1)2 (1.84g, 12.7 mmol) in water (40ml).
Stirring was continued for 1 hr after which water (40 ml) was added. The solution was extracted with CH2Cl2 (4 x 30ml) and the organic layers washed
with 10% NaHCO3 followed by an aqueous wash. After drying with MgSO4 and evaporating the CH2Cl2 the crude product was distilled affording l-menthone
(2.89 g, 98%). The spectra (ir and nmr) were identical with those of authentic material{9,10}.
Oxidation of primary alcohols under identical conditions gave an aldehyde only in the case of benzyl alcohol{11}. Other primary alcohols gave esters
as tabulated in table 2. This table also includes our results on the oxidation of of ethers to esters. Though the yields were not nearly as good as
for the alcohols, the data is reported because of the unusual and potentially useful transformation{12}. The ethers were oxidized under similar
conditions as the alcohols except that the reactions were carried out at room temperature for from 4-16 hrs. Heating does not seem to increase the
yield.
We are presently carrying out studies to improve the yields on the ether to ester transformation and to utilize the 1deg alcohol oxidation for the
preparation of lactones from a-w diols.
______________________________________________________________________________
Table 2. Oxidation of 1 deg-Alcohols and Ethers Using Calcium and Sodium Hypochlorite.
Run Substrate Product %Yield(a) (Ca(OCl)2 NaOCl) Reference
--------------------------------------------------------------------------------------------------------
1 benzyl alcohol -> benzaldehyde 98 98 10
2 1-pentanol -> pentyl pentanoate 83 91 10
3 l-hexanol -> hexyl hexanoate 98 98 9e
4 3-methyl butanol -> 3-methyl butyl isovalerate 76 87 10
5 ethyl alcohol -> ethyl acetate -- (b) 9b, 10
6 ethyl alcohol -> ethyl acetate (b) -- --
7 butyl ether -> butyl butanoate 40 -- 10
8 tetrahydrofuran -> g-butyrolactone 68 -- 9b, 10
9 tetrahydropyran -> d-valerolactone 56(c) -- 9b, 10
(a) Isolated yield
(b) Yield not calculated due to the volatility of the products but significant conversion was indicated by IR and NMR analysis.
(c) Yield obtained by GC analysis."
I am very busy at the moment but I am very grateful for everyones help and will be on later to analyze all this new information.
[Edited on 17-2-2015 by RelativeEffectiveness]
[Edited on 17-2-2015 by RelativeEffectiveness]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Could you two stop insulting each other? Chemo's calculating percent theoretical yield based on mass product per expected mass product from limiting
reagent, turd's calculating practical yield based on mass product per mass substrate assuming excess other reagents. Neither of you two is a
"wikipedia chemist" nor completely lacking practical experience, for the love of common sense.
If the OP is genuinely interested in chemistry, Chemo's way is the one to understand first.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Yes you can. Even if you had the intention of making the drug that wouldn't be against forum rules, as long as you're rather discussing it in a
scientific manner and not just asking for a "recipe".
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
I apologize for the bickering. You all have been extremely helpful. Im in search of a website (or any reference format) that would be ideal for
helping me re-learn how to calculate the stoichiometry of chemical equations. As a few of you have already pointed out, this particular equation is
not the ideal starting point. However, I had been dying to understand it for quite some time and now that Ive reverted from a life of persistent
neurotransmitter disarray in my brain to one of pursuing the comprehension and application of chemistry, I can assure you that my intentions are
genuine (not that this is a requirement). This is a great forum and I plan on sticking around for the copious amounts of applicable discussion that
make this forum an excellent resource and an equally beneficial tool. If anyone knows of resource that might be of help in re-learning how to
calculate the stoichiometry of equations I would be much obliged!
[Edited on 17-2-2015 by RelativeEffectiveness]
|
|
|
RelativeEffectiveness
Harmless

Posts: 6
Registered: 10-2-2015
Member Is Offline
Mood: No Mood
|
|
Thank you especially to Chemosynthesis, turd and Darkstar. Darkstar, that was great of you to take the time to arrange that. That was amazingly
insightful.
However, Im still at a loss as to what the stoichiometry would look like. Are the THF and the Ca(OCl)2 both 1 and 1? And I assume the limiting reagent
is THF? Or would it depend on the intent of thr reaction and could also be Ca(OCl)2? Id like to understand and see how the acetonitrile and acetic
acid play a role in the reaction.
[Edited on 17-2-2015 by RelativeEffectiveness]
[Edited on 17-2-2015 by RelativeEffectiveness]
|
|
|
| Pages:
1
2 |