| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Gallium Nitrate
Would anyone have any pointers to discover the Heat of Formation of Gallium Nitrate Ga(NO3)3 ?
I tried Nist a few times, and also some other refs, and came up with nothing.
Ga only seems to react with HNO3 when heated to about 110C, so i expect the delta Hf to be rather small.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Did i ask the Wrong question ?
Is Gallium Nitrate a well-known no-no ?
Is the DEA going to beat down my door any minute now ?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
No, it's just gallium salts aren't often experimented with due to the high price of gallium metal. What are your plans for the gallium nitrate?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Ga(NO3)2 Hf
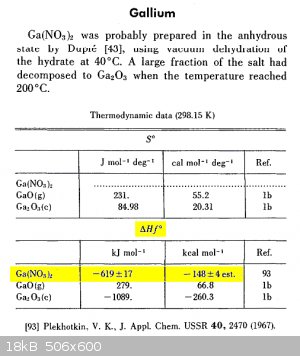
.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thank you Franklyn.
Not sure if -629 or -148 applies.
Probably -148 as it needs so much heat to react with HNO3
Will calculate when sober.
I have no Plans for it.
Just had some Gallium, and thought it time to see what it does.
A quick google says that Gallium Nitrate interferes with Calcium mechanisms in humans, so Gloves then, before stuffing it into a bottle after a few
recrystallisations.
[Edited on 6-10-2014 by aga]
[Edited on 6-10-2014 by aga]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Sounds interesting!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Seems (from the googling) Gallium Nitrate is sometimes used to reduce the rate at which Calcium is taken from Bone into the Blood (later excreted).
Dunno the mechanisms, but the googling said cancer sufferers sometimes have a lot of Bone Loss, so they use Gallium Nitrate to mitigate that.
Best ignore My googling and go google.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ aga
" Will calculate when sober "
— Yeah do that first.
" Not sure if -629 or -148 applies."
— Units are indicated just above each entry
-619 kilojoules / mol , -148 kilocalories / mol
If you know one you don't need the other. Multiply or divide by 4.186
High Temperature Properties & Decomposition of Inorganic Salts : Nitrates & Nitrites
Attachment: Nitrates & Nitrites Hi Temp Proper & Decomp of .pdf (2.6MB)
This file has been downloaded 710 times
.
[Edited on 7-10-2014 by franklyn]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ah yes.
This morning with my glasses on i see the titles now.
So the delta H is +59 kJ/mol.
That explains it.
Many Thanks for the pdf and the Assitance.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Huh? Which delta H? Explains what?
The speaking in tongues is much discouraged here. 
Gallium is of course most famous for dissolving aluminium very quickly (see a Nurdrage Utoob on it). The resulting alloy is very reactive and
interesting to play with.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
2 Ga + 6 HNO3 => 2 Ga(NO3)3 + 3 H2
(0 + -148) - (-207 + 0) = 59 kJ/mol
er, enthalpy change, er, delta H, er, heat of reaction ... (blush) not sure sir.
also not sure if the 2, 6, 2 or 3 come into it either.
Verily shalt my bodkin be shriven.
Anyway, it won't go without some heating.
Strangely the beaker contents were Green this morning, after being left to stand (unheated) overnight.
Some stirring and it's gone clear again.
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have made gallium nitrate by dissolving gallium in diluted nitric acid (about 20%) I had no special problems though it required steady warming and
took quite a long time. With stronger acid the reaction becomes more exothermic and self heats and after a while tiny micro explosion occur in the
molten gallium. I found it difficult to free the gallium nitrate of nitrate acid and it is also rather deliquescent.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | 2 Ga + 6 HNO3 => 2 Ga(NO3)3 + 3 H2
(0 + -148) - (-207 + 0) = 59 kJ/mol
er, enthalpy change, er, delta H, er, heat of reaction ... (blush) not sure sir.
also not sure if the 2, 6, 2 or 3 come into it either.
|
That reaction would be for gallium with 100 % HNO3 and not gallium + dilute nitric acid (remember?)
What's more, strong (but not anhydrous) HNO3 with gallium almost certainly leads to some oxidation by the nitrate ions (and not only by protons):
NO<sub>3</sub><sup>-</sup> + 4 H<sup>+</sup> + 3 e<sup>-</sup> === > NO + 2 H2O
So the basic premises for Delta H Reaction calculation appear to be off...
[Edited on 7-10-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So you're accusing me of not knowing what i'm doing ?
Not having a Clue ?
Being totally Ignorant regarding Enthalpy ?
If so, you're completely right.
Could you please walk through how you'd calculate for this reaction ?
Pretty please ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It's difficult here because two reactions take place and we don't know the ratio of them, For dilute nitric acid:
1) Deprotonation of nitric acid:
HNO3 + H2O === > H3O+ + NO3- (all (aq))
If dilute NA was used this reaction is already in the PAST and reaction enthalpy has already been accounted for.
2) Oxidation of Ga with H3O+ and solvation of Ga3+:
Ca(s) + 3 H3O+(aq) === > Ga3+(aq) + 3/2 H2(g) + 3 H2O(l)
3) Oxidation of Ga with H3O+ and NO3(-) and solvation of Ga3+:
Ga(s) + NO3(-)(aq) + 4 H3O+(aq) === > Ga3+(aq) + NO(g) + 6 H2O(l)
---------
But because we don't really know the ratio in which 2) and 3) occur it's hard to determine Delta H...
[Edited on 7-10-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The sensation is almost the same as being fascinated by a Candle Flame, then Bright Sunlight arrives.
Learn more i must.
Best make some 99.99% nitric and do it all again to see what it does with little or no water.
There was quite a bit of NO2 when heating during the reaction, and definitely copius volumes of NO when heating after.
I'll have a word with my Dark Master and ask about how the Enthalpy maths thing works.
Thanks to all for their input and assistance.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | I found it difficult to free the gallium nitrate of nitrate acid and it is also rather deliquescent. |
Me too.
Day 2 and still trying to boil off the acid.
I found that adding water helps.
Maybe not helping get rid of the acid, but helps See it boiling off.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Is this stuff safe? Totally fascinating stuff. Your going to have to explain the enthalpy thing to me at some point  . School finally gave me some old GCSE (Scottish equiv) text books because the
syllabus has changed so they not needed. They gave me 3 today, but to be honest they are shit compared to the other books I have. . School finally gave me some old GCSE (Scottish equiv) text books because the
syllabus has changed so they not needed. They gave me 3 today, but to be honest they are shit compared to the other books I have.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Safe? No idea.
I just had some Gallium and some Nitric acid.
Shoved them in a beaker, heated and got some Other stuff.
At this point i would like to know the Enthalpy, and will entreat the Gods to discover how to calculate such Divine things.
Chickens everywhere Beware ! Run ! Hide !
To glean this Forbidden Power, I must cast the steaming bowels of a Happy Virgin Chicken before my chosen God, as is the Custom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Best make some 99.99% nitric and do it all again to see what it does with little or no water.
|
For dissolving metals in NA, dilute NA is far better. Depending on the reactivity of the metal, 35 to 70 w% usually.
I understand your quest to find the enthalpy of reaction here but it's one of these cases where in practice it's not so important. Although there is
considerable heat being released (try a small piece of copper in 35 % NA and measure it!) these reactions tend to be fairly slow and the heat is
generated gradually. That makes thermal runaways less likely, although with alkali metals or aluminium (as well as some others) it's still possible
depending on concentration, amounts of metal and physical form.
Electropositive metals will generally generate less NO (relying more on H3O+ as oxidiser) but metals like Cu, Ag, Pb depend on the oxidising by NO3- +
H3O+. That's what makes nitric acid so useful for dissolving these metals.
Quote: Originally posted by aga  |
At this point i would like to know the Enthalpy, and will entreat the Gods to discover how to calculate such Divine things.
|
We'll do this in private.
[Edited on 8-10-2014 by blogfast25]
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
If it is difficult to boil off the HNO3 from the gallium nitrate, perhaps instead of directly reacting HNO3 with gallium, react the HNO3 with some
gallium compound.
Add Na2CO3 solution to your liquid until all the gallium ions precipitate as Ga2(CO3)3.
Then filter the Ga2(CO3)3. Add the exact quantity of HNO3 needed.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by blogfast25  | | That makes thermal runaways less likely, although with alkali metals or aluminium (as well as some others) it's still possible depending on
concentration, amounts of metal and physical form. |
Aluminum doesn't react with HNO3. It forms a passivation layer.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
]Pedantic mode On[
So it *does* react with Al (forming the passivation layer) yet in a Real experiment you'd not see much going on because that layer would stop further
reaction happening, unless you used nanometre-sized Al particles, in which case you'd see something happening for short time longer.
]Pedantic mode off[
My Ga(NO3)3 is still a clear liquid.
Way more than stoichiometric amount of HNO3 in the pot.
My Brilliant Plan is to try to boil it off until either it's Gone or i forget what it is that is also in the pot.
[Edited on 10-10-2014 by aga]
|
|
|
eidolonicaurum
Hazard to Self
 
Posts: 71
Registered: 2-1-2014
Location: Area 51
Member Is Offline
Mood: Hydric
|
|
Quote: Originally posted by aga  |
Strangely the beaker contents were Green this morning, after being left to stand (unheated) overnight.
Some stirring and it's gone clear again.
|
I tried this exact same experiment myself a couple of years ago, and got the same result. I used concentrated nitric acid, and got a dark green
solution which appeared to release nitrogen dioxide when it was exposed to the air, e.g. through stirring or shaking. The picture below is what I
generated. It turned totally colourless when all the nitrogen dioxide was allowed to escape.

I also noticed that when the gallium was in the process of being dissolved, a dark brown solid formed on the surface. This also dissolved as the
reaction progressed, however, I don't have a picture of this. I was unable to find any reference to this on the web at the time, and it would be
interesting if anybody could replicate this result.
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The green is most likely from N2O3, formed by reduction of nitric acid.
|
|
|
| Pages:
1
2 |