scrubs2009
Harmless

Posts: 22
Registered: 7-12-2014
Member Is Offline
Mood: No Mood
|
|
Tetraamminecopper(II) Persulfate production.
Hello all, I am experimenting with various types of pyrotechnics and am attempting to make Tetraamminecopper(II) Persulfate. I already have the liquid
Tetraamminecopper(II) Persulfate and I was wondering if it would be safe to boil it down instead of waiting for evaporation?
(For safety I am only making a very small amount, about 10ml)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I wouldn't heat it as ammonium persulfate alone decomposes already at 120C according to wikipedia. The copper probably has a catalytic effect and
might lower this decomposition temperature a lot. This is generic advice as I don't have any experience with the compound specifically.
What procedure did you use to prepare it?
|
|
|
Bert
|
Thread Moved
22-12-2014 at 12:46 |
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
What I'm fairly sure you have is not liquid tetramminecopper(II) persulphate, but aqueous tetramminecopper(II) persulphate. I'd just let it
evaporate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
I don't think boiling it down or letting it evaporate will result in you isolating any Tetraamminecopper(II) Persulfate. The ammonia ligands will just
evaporate away in either case. You would be better off trying to precipitate it by adding ethanol to the solution, in the manner that
Tetraamminecopper sulfate is isolated. It can also be obtained by evaporating under a stream of dry ammonia, but I doubt that's a viable option for
you.
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
I just made some of this the other day.
You can precipitate out the tetraamminecopper(II) persulfate out by cooling the solution in your fridge to 0 degrees C. It forms nice needle-like
crystals.
Also, be wary of decomposition during the drying of the compound, as it has a tendency to decompose if left out in the air for too long. However, if
you keep an eye on it and store immediately once it dries, decomposition should be minimal to non-existent.
Here are some pics from my synth:
The solution prior to precipitation

The crystals left after cooling/decanting

Final product after drying

BLaRgISH
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Well done! I stand corrected
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
How can this compound be synthesized?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
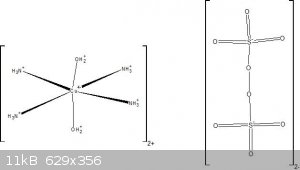
I managed to synthesize this compound. I also made an attempt to draw this compound's structure in ChemSketch  : :
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|