| Pages:
1
2
3
4
..
6 |
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | Chris:
That Azurite preparation method by Brauer could be carried out successfully in a PET C*ke bottle.
This here fellow pumps up a PET drinks bottle to 120 psi with CO2 without blinking: that’s about 8 bar. Other references confirm these values.
|
I have had such bottles pop from wine fermentation, believe it or not, so while they will probably take the pressure, you may want to put some duct
tape around the bottle just in case.
I wonder if a champagne bottle would take more pressure than that (of course, if it shatters, you get high velocity broken glass, so wrapping it with
tape is a must, and even with tape, terrifying).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CHRIS25  | | That's a whole lot of unfamiliar maths, but the idea is something I would like to try. Just need to find fizz giz tops and a CO2 extinguisher for dry
ice. Thankyou for the links Gert, something new. |
What would you want the dry ice for? Here the CO2 and pressure are generated in situ, by the reagents. No extra CO2 needed.
Quote: Originally posted by DraconicAcid  | I have had such bottles pop from wine fermentation, believe it or not, so while they will probably take the pressure, you may want to put some duct
tape around the bottle just in case.
I wonder if a champagne bottle would take more pressure than that (of course, if it shatters, you get high velocity broken glass, so wrapping it with
tape is a must, and even with tape, terrifying). |
Where these genuine OEM PET bottles? I had some delamination problems with bottles from a ‘starter’ beer making kit but these clearly weren’t
OEM (nor did they 'pop'). And was that CO2 from primary or secondary fermentation?
Glass, when well utilised can take much more pressure than flimsy PET carbonated drinks bottles but as you point out, exploding glassware is no joke.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by CHRIS25  | | That's a whole lot of unfamiliar maths, but the idea is something I would like to try. Just need to find fizz giz tops and a CO2 extinguisher for dry
ice. Thankyou for the links Gert, something new. |
What would you want the dry ice for? Here the CO2 and pressure are generated in situ, by the reagents. No extra CO2 needed.
|
My thinking was to test any bottle to see how much pressure it could take first.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  |
Where these genuine OEM PET bottles? I had some delamination problems with bottles from a ‘starter’ beer making kit but these clearly weren’t
OEM (nor did they 'pop'). And was that CO2 from primary or secondary fermentation? |
The one incident that I remember was a batch of plum wine that my mother was making- the batch was slightly larger than the carboy, so she put the
remainder into a pop bottle, intending to let off the pressure frequently (which I do all the time). She forgot and went on vacation for a couple of
days- when she came back, the bottle had ruptured, knocking a great chunk out of the carboy next to it. What a horrible mess, and the loss of gallons
of wine....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | | The one incident that I remember was a batch of plum wine that my mother was making- the batch was slightly larger than the carboy, so she put the
remainder into a pop bottle, intending to let off the pressure frequently (which I do all the time). She forgot and went on vacation for a couple of
days- when she came back, the bottle had ruptured, knocking a great chunk out of the carboy next to it. What a horrible mess, and the loss of gallons
of wine.... |
For 1 L of 13 ABV wine, about 125 g of CO2 are produced. If you tried to contain that you'd exceed the recommended carbonation levels of 8 g CO2/L
enormously and things would definitely go pop. I had a water lock blown off a glass demijohn in my debutante brewing days, presumably due to partial
blockage of the lock.
But anything in the range of 8 to 12 g/L CO2 must be safe, taking into account bottle manufacturers will build a safety factor into their products.
[Edited on 29-11-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Images from tests
Maybe this thread has run dry but I thought I would at least post some images, comments would be helpful if anyone has anything further to add:
The Paint trial: Left hand watercolour paper painted with the blue copper carbonate and the right hand painted with the green. (Powdered down and
mixed with linseed oil). As opposed to the right side which is stable, fixed and does not wash or rub away, the left side blue turned to powder and
came off (after 15 hours) leaving a slightly darker green underneath?

The Blue and yellow containers (copper sulphate and sodium carbonate): Blue container contains the 'cake' from the "No NaOH added" (bad photo but is
greener) solution and the yellow container contains the "Added 1 mol NaOH" (clearly blue) solution

The Sodium BIcarbonate and copper sulphate:
No explanation, colour obvious.

‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Chris:
Since as you are looking for greenish copper based pigments, presumably for chalk paints, there's another one that's easy to prepare: dicopper
chloride trihydroxyde, or Cu<sub>2</sub>Cl(OH)<sub>3</sub>:
http://en.wikipedia.org/wiki/Dicopper_chloride_trihydroxide#...
I prepared this from CuSO4, NaCl and NaOH:
2 CuSO4(aq) + NaCl(aq) + 3 NaOH(aq) === > Cu2Cl(OH)3(s) + 2 Na2SO4(aq)
Add the NaOH solution slowly to the 2 CuSO4 + NaCl solution, with constant stirring. Add only a stoichiometric amount of NaOH: overshooting converts
the greenish blue dicopper chloride trihydroxyde precipitate to blue Cu(OH)<sub>2</sub>.
I didn't actually characterise the obtained precipitate but I did test the carefully washed product for bound chloride and there was plenty of that.
[Edited on 29-11-2014 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CHRIS25  | The Paint trial: Left hand watercolour paper painted with the blue copper carbonate and the right hand painted with the green. (Powdered down and
mixed with linseed oil). As opposed to the right side which is stable, fixed and does not wash or rub away, the left side blue turned to powder and
came off (after 15 hours) leaving a slightly darker green underneath?
|
Again, I suspect inadequate drying may cause the poor adhesion on the left hand side. Better drying of ANY of these products should also reinforce the
green hues, not the blue tones.
I suggest after washing with water and careful dripping 'dry', to wash the filter cake a few times with small amounts of acetone or clear methylated
spirits (or even clear methanol). Then dry at RT (or below 50 C in any case) to drive of the solvent, then grind to required fineness and give it a
final dry for a few days in a CaCl2 desiccator.
'Small amounts of moisture + linseed oil = problems', I think, because oil and water don't mix.
[Edited on 29-11-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Actually I totally agree, plus I predicted that a copper carbonate with water molecules would not work. But amazingly, the green carbonate from a
year ago was quite moist and the blue carbonate from a year ago was totally dry due to it being easily powdered and well, (non-scientifically)
certainly more dry than the green which was clearly "wet". Anyway the green was what turned out to be quite good. So I am very thankful for the
above links, and will get onto that right away. (Actually I am experimenting with all this just for the paint pigments this time, not for the chalk
paint which was the calcium carbonate from last week). I appreciate the links and information. (At least the yellow pigment works), though could be
better with a buchner flask
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I’ve prepared some of what I believe to be Cu<sub>2</sub>Cl(OH)<sub>3</sub>, from a mixture of CuSO4, NaCl and NaOH.
0.1 mol of CuSO4.5H2O and 0.2 mol of NaCl were dissolved in 150 ml water. While stirring magnetically 0.1 M NaOH was slowly added. A light green
precipitate formed but also a few specs of dark blue presumed to be Cu(OH)2. On further stirring these disappeared completely. I didn’t add the full
stoichiometric amount of NaOH to avoid further Cu(OH)2 formation (previous experience showed that the precipitate converts to Cu(OH)2 with excess
alkali). Here it is after settling:

The remainder of Cu<sup>2+</sup> can be seen in the supernatant.
The precipitate filtered and washed with difficulty, slow even with a Buchner. A final rinse with a bit of acetone removed most of the wash water.
The acetone was evaporated on the hot plate, low setting. Here’s the product:

It’s already much greener than the original precipitate.
I’m now drying it in an oven at a modest 90 C, hopefully to constant weight. A preliminary test showed it’s much more resistant to heat than
Cu(OH)2 but it’s still possible I end up with a black product of mainly CuO.
If it holds up this green colour I’ll probably determine Cu content to compare it to the theoretical value of
Cu<sub>2</sub>Cl(OH)<sub>3</sub>.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I sense a range of colours looming - a Chemical Palette ...
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
So far I have prepared Cu carbonate in 4 different ways, and four different shades ranging from complete blue, to blue-green turquoise and dark green.
The good news is that when the dried compound is mixed with linseed oil it always turns to the same colour green and paints on water colour paper
very well indeed. I wish I could grind it a bit more but seems impossible without a mechanical device. I will be doing Blogfasts's recipe today and
then I want to be able compare the copper and chloride amounts with the copper oxychloride I bought from a farm supply.
So using Blogfast's mole ratios: I had quite a fair amount of blue globulars spinning around with the magnetic stirrer and although the precipitate
was turquoise when I had finished adding the NaOH, I decided to leave it for a few hours and now it is quite clearly green, I will be interested to
see whether or not there is any copper hydroxide precipitate mixed in with this.
(NOTE: THE 'SUPER' TEXTS BUTTON ARE NOT WORKING? NO IDEA WHY TRIED 4 TIMES)
So: Cu+2SO-2 + 2Na+Cl- + Na+OH-
can provide us with either: Cu(OH)2 or Cu2(OH)3Cl all dependent upon how fast one adds the NaOH, assuming that
there are enough free Cl- in solution?
I see that Na+ replaces Cu2+ in solution to give sodium sulphate, (reactivity series), but I still want to understand why the
Copper and the chloride and the hydroxide come together as they do, and what prevents CuCl2 forming instead? - stupid not thinking, because
one needs H ions
an acidic environment for this.

[Edited on 8-12-2014 by CHRIS25]
[Edited on 8-12-2014 by CHRIS25]
[Edited on 8-12-2014 by CHRIS25]
[Edited on 8-12-2014 by CHRIS25]
[Edited on 8-12-2014 by CHRIS25]
[Edited on 8-12-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
My product reached constant weight after about 1 h @ 90 C. I kept drying for another 1/2 h and another 1/2 h, getting identical weight readings. The
colour is now very much like my last photo: green, not green/blue or blue/green. It's waiting in a CaCl2 desiccator for the Cu determination.
I also tested a small piece at about 200 C and it fell apart to CuO + HCl + H2O and turned black. This is in agreement with Wiki's entry (even though
it wrongfully mentions an MP of 250 C!).
Quote: Originally posted by CHRIS25  | (NOTE: THE 'SUPER' TEXTS BUTTON ARE NOT WORKING? NO IDEA WHY TRIED 4 TIMES)
So: Cu+2SO-2 + 2Na+Cl- + Na+OH-
can provide us with either: Cu(OH)2 or Cu2(OH)3Cl all dependent upon how fast one adds the NaOH, assuming that
there are enough free Cl- in solution?
|
Chris, you need to use the angular brackets ( < and > , not the rectangular
ones ( [ and ] ) to embed the sub and sup tags. , not the rectangular
ones ( [ and ] ) to embed the sub and sup tags.
A mixture of CuSO4 and NaCl in solution is an 'ion soup' in water. Besides water molecules, slightly simply put it contains
Cu<sup>2+</sup>, Na<sup>+</sup>, SO<sub>4</sub><sup>2-</sup> and Cl<sup>-</sup> ions. The
reactivity series doesn't enter into it because dissolving the salts causes no reduction or oxidation anywhere.
The Cu(OH)2 seems to form briefly when locally high OH<sup>-</sup> concentration is reached during the NaOH solution addition.
But as long as the supernatant solution is neutralish AND still contains Cl<sup>-</sup> ions, the Cu(OH)2 is converted to
Cu2Cl(OH)3:
2 Cu(OH)2(s) + Cl<sup>-</sup>(aq) ===> Cu2Cl(OH)3(s) + OH<sup>-</sup>(aq)
This makes me believe the chlorohydroxide is even more insoluble than the straight hydroxide.
CuCl2 cannot form because its (quite high) solubility limit has not been reached.
[Edited on 8-12-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Another case of Aluminium sulphate - darn tough to drive off the water. Anyway since each Cl ion requires 2 Cu ions to make
Cu2(OH)3Cl; the 1 mol Copper sulpate supplied is the limiting reagent in terms of Cu ions and therefore can only yield 0.5 mol
of the Cu2(OH)3Cl. Expected yield was therefore 10.68g. Actual yield is 5.96g, but am allowing for 1g loss on the filter paper
(very sticky and tough to scrape off when so wet) plus maybe a 2g loss through incomplete reaction since there was some copper hydroxide mixed in with
the precipitate. So a 3 gram loss is quite feasible here, close then to the 10gram expected yield.
You did not mention your yield Gert?
Re the HTML text, I always use the buttons and never use the actual HTML code when inserting the sub and super scripts.
So now to compare this with copper oxychloride which both Wiki and ChemSpider say are exactly the same.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CHRIS25  | Another case of Aluminium sulphate - darn tough to drive off the water. You did not mention your yield Gert?
[snip]
Re the HTML text, I always use the buttons and never use the actual HTML code when inserting the sub and super scripts.
|
Hmm. The difficulties of getting rid of water in aluminium sulphate hydrate and wet Cu2Cl(OH)3 are very different in nature. Cu2Cl(OH)3 fresh
precipitate e.g. isn't a hydrate, it's simply a wet crystalline substance that's insoluble in water.
I didn't bother with yield because of too much product loss during filtration difficulties. Next time I'll use mainly decantation to wash the product.
What did you do?
Interesting observation: those bits that had gone black at approx. 200 C have regained their original colour, pointing to thermochromism rather than
decomposition at 200 C. That males the chloridehydroxide much more resistant to dehydration than Cu(OH)2.
I didn't even know there were such buttons, haha!
[Edited on 9-12-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
I allowed it to filter overnight - 12 hours. Then scraped the single solid wet mass into a dish, put it into my homemade teapot oven, kept the
temperature between 55 to 90c, (can't control the temp very well in a teapot! After regular checking and weighing, a total of 8.52g of water was
driven away after 2 hours 45 mins interspersed with occasional breaking up of the mass.
....yes those buttons save time
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
12 hours? Ouch. There has to be a way to improve the filtrability of that stuff...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Azurite in a PET bottle?
Following Chris' link to a Brauer referenced method of preparing Azurite, here:
http://books.google.co.uk/books?id=Pef47TK5NfkC&pg=PA102...
... I've started a small experiment to that effect.
In a former carbonated drinks PET bottle of 600 ml volume, 0.3 mol of Cu(NO3)2 dissolved in about 100 ml of water and 90 g of small (0.5 - 1 cm)
chunks of clean limestone (about 3 times the stoichiometric requirement) were loaded:

Fizzing started immediately but I believe this is mainly due to small amounts of residual nitric acid (I had to prepare the Cu(NO3)2 somewhat ad hoc).
So for the next couple of hours I'll release pressure every now and again.
Then I will allow reaction to take place for about a week (it's really cold here and the bottle has to stay outside for obvious reasons).
Calculations show that on stoichiometric conversion pressure in the bottle should be about 5 - 8 bar.
[Edited on 13-12-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
On that link just scroll down a couple of times.
@Gert: Calculations show that on stoichiometric conversion pressure in the bottle should be about 5 - 8 bar. Something I need to understand but am
too scared to ask.......
[Edited on 13-12-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CHRIS25  | On that link just scroll down a couple of times.
@Gert: Calculations show that on stoichiometric conversion pressure in the bottle should be about 5 - 8 bar. Something I need to understand but am
too scared to ask.......
[Edited on 13-12-2014 by CHRIS25] |
I'm no sure I follow? Scroll down to what?
Re. pressure. When you're topping up a sagging tyre you're adding gas without substantially changing the volume (or the temperature) of the tyre. The
pressure increase that results is governed by the Ideal Gas Law: http://en.wikipedia.org/wiki/Ideal_gas_law
In the case of our reaction we are adding gas to the bottle because the reaction generates CO2, namely 1 mol per every 3 mol of
Cu(NO3)2, at least stoichiometrically speaking.
Since as I know how much Cu(NO3)2 I used (0.3 mol) and what the volume of the bottle is (600 ml, but strictly speaking I need to
account for the volume taken up by the reagents: water, Cu(NO3)2 and limestone) I can use the Ideal Gas Law to predict the end
pressure in the bottle. I designed the experiment to achieve 5 - 8 bar, as prescribed in the method you linked to.
I'm increasingly convinced that Azurite and Malachite are part of one equilibrium:
2 Cu3(CO3)2(0H)2(s) [Azurite, blue] + H2O(l) <===> 3 Cu2CO3(OH)2 [Malachite, green](s) + CO2(g) .... Eq.1
... so that one can be obtained from the other, depending on actual conditions.
The bottle now feels 'hard' due to pressure and there is unmistakably some blue precipitate already formed.
I believe the formation of Azurite may proceed as follows:
1st step:
Formation of Malachite according to:
6 Cu(NO3)2 + 6 CaCO3 + 3 H2O === > 3 Cu2CO3(OH)2 + 6 Ca(NO3)2 + 3 CO2 .... Eq.2
2nd step:
Conversion of the Malachite to Azurite, basically as per Eq.1 (but in reverse):
3 Cu2CO3(OH)2 + CO2 === > 2 Cu3(CO3)2(OH)2 + H2O
Add this to Eq.2 for the overall reaction equation:
6 Cu(NO3)2 + 6 CaCO3 + 2 H2O === > 2 Cu3(CO3)2(OH)2 + 6 Ca(NO3)2 + 2 CO2
and divide by 2:
3 Cu(NO3)2 + 3 CaCO3 + H2O === > Cu3(CO3)2(OH)2 + 3 Ca(NO3)2 + CO2
So the conversion of the first Malachite to Azurite indeed uses the CO2 generated in step 1 because we don't allow it to escape.
Quite neat...
[Edited on 14-12-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Thought maybe you might find this interesting: What I find interesting are the 2 different oxidation states of copper and the way they are bonded.
But I am probably stupid for asking (who cares) would this be the same difference in the blue and the green carbonate made synthetically. (don't mean
the turquoise), I mean literally the green I made and the totally blue that I made?
Azurite Copper carbonate hydroxide
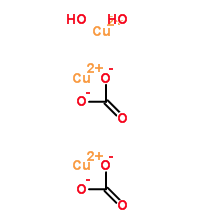
Malachite
Carbonic Acid copper hydrate
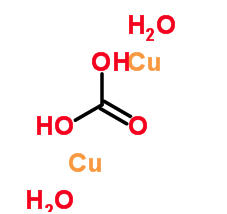
[Edited on 15-12-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | So the conversion of the first Malachite to Azurite indeed uses the CO2 generated in step 1 because we don't allow it to escape.
|
Does that mean that the CO2 mostly escapes in the Malachite reaction due to sheer Kinetics, or does the increase in pressure also affect
the thermodynamics enabling the Azurite reaction to happen ?
Presumably Malachite would become Azurite due to atmospheric CO2 over time otherwise.
[Edited on 15-12-2014 by aga]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Aga, from what I deduce so far (and am always wrong in my deductions...) the azurite blue is the Pure copper carbonate while the green is impure, so
to speak. Also the green does not turn blue and the blue does not turn green, otherwise all my three samples from a year ago would have changed colour
by now. All I can say is that whenever I mix the blue carbonate with Linseed oil, wet brushes on paper, it immediately turns green, even when the
blue is applied to the paper it does not take long for it to change colour to green. These are only my observations though. I still need to know
whether the molecular structures above that I found apply to the rocks only or also the synthetically made product at home.
Should also have added that when I used Sodium carbonate with copper sulphate AND NaOH I got a blue copper carbonate.
When the same mix was done without the NaOH I got a green carbonate. What is interesting is the fact that I noticed Half as much Carbon dioxide being
driven off with the NaOH add into the equation. This then at first glance looks like with less carbon dioxide being driven off, (Since I used exactly
the same quantities in all the experiments, half of it must have been used and remained in the reaction, I don't know enough chemistry at the moment
to understand all this.
[Edited on 15-12-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Is the Paper treated with anything like a Sizing agent ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
OK. Lots of interesting questions here, so let me try.
Quote: Originally posted by CHRIS25  | | Thought maybe you might find this interesting: What I find interesting are the 2 different oxidation states of copper and the way they are bonded.
But I am probably stupid for asking (who cares) would this be the same difference in the blue and the green carbonate made synthetically. (don't mean
the turquoise), I mean literally the green I made and the totally blue that I made? |
The oxidation state of Cu in BOTH Azurite and Malachite is +2, so that cannot explain the difference in colour. Cu(I) is only really quite stable in
Cu<sub>2</sub>O, copper(I) oxide, which is kind of Bordeaux.
BTW, that second structure labelled 'carbonic acid copper hydrate' is simply baloney. Plain wrong. The real structure involves
Cu<sup>2+</sup> ions, CO<sub>3</sub><sup>2-</sup> ions and OH<sup>-</sup> ions and no water molecules.
Going by that structure the copper wouldn't even carry any charge at all!!!
Quote: Originally posted by aga  | Quote: Originally posted by blogfast25  | | So the conversion of the first Malachite to Azurite indeed uses the CO2 generated in step 1 because we don't allow it to escape.
|
Does that mean that the CO2 mostly escapes in the Malachite reaction due to sheer Kinetics, or does the increase in pressure also affect
the thermodynamics enabling the Azurite reaction to happen ?
Presumably Malachite would become Azurite due to atmospheric CO2 over time otherwise.
[Edited on 15-12-2014 by aga] |
The formation of Malachite (from copper nitrate and calcium carbonate) generates lots of CO2, as per the equation. If we allowed that CO2 to escape
(open container) then nothing further would happen but if we ‘keep’ the CO2 then:
3 Cu2CO3(OH)2 + CO2 === > 2 Cu3(CO3)2(OH)2 + H2O
… occurs. I can kind of see the green Malachite being converted to the blue Azurite, just looking at the bottle.
Azurite at atmospheric conditions is probably so-called meta-stable: thermodynamically it SHOULD release that CO2 again (at least in the presence of
water) but it may only do this very slowly, so it APPEARS to us to be stable.
But atmospheric CO2 is of far too low pressure to cause that conversion (Malachite to Azurite) and indeed all copper hydroxycarbonates we see on
statues and other copper structures are always green, never blue (even though it’s subjective, colours always are).
Quote: Originally posted by CHRIS25  | Aga, from what I deduce so far (and am always wrong in my deductions...) the azurite blue is the Pure copper carbonate while the green is impure, so
to speak. Also the green does not turn blue and the blue does not turn green, otherwise all my three samples from a year ago would have changed colour
by now. All I can say is that whenever I mix the blue carbonate with Linseed oil, wet brushes on paper, it immediately turns green, even when the
blue is applied to the paper it does not take long for it to change colour to green. These are only my observations though. I still need to know
whether the molecular structures above that I found apply to the rocks only or also the synthetically made product at home.
|
It’s interesting that your blue turns green so quickly on wetting. I’ll be checking that with the Azurite, once it's come off the high pressure
CO2 diet.
[Edited on 15-12-2014 by blogfast25]
|
|
|
| Pages:
1
2
3
4
..
6 |